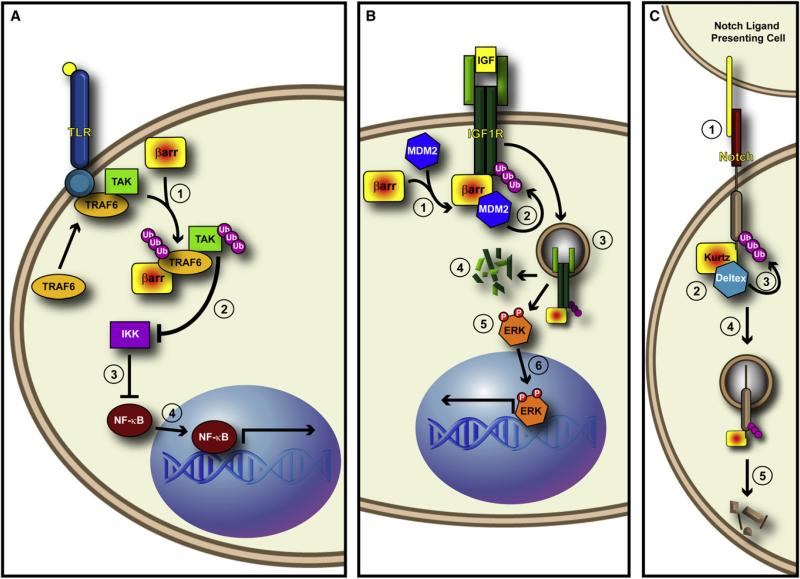
Figure 4. β-arrestins Are Multifunctional Signaling Adaptors.
(A) β-arrestin promotes the ubiquitination of TRAF6. (1) Upon binding of ligand to the Toll-like Receptor, β-arrestin binds to and promotes the autoubiquitination of TRAF6. This autoubiquitination event is required for downstream activation of TAK and subsequent activation of NF-κB signaling. (2) It may be that β-arrestin-mediated ubiquitination of TRAF6 leads to activation of TAK, which inhibits IKK, (3) thus freeing NF-κB from IKK-mediated inhibition. (4) This would then allow NF-kB translocation into the nucleus and activation of transcription.
(B) β-arrestin acts as an E3 ligase adaptor in response to IGF stimulation. (1) After IGF binds to the tetrameric IGF1R, β-arrestin recruits Mdm2 to the receptor. (2) Mdm2 ubiquitinates IGF1R, thus (3) leading to its internalization. (4) Once internalized, IGF1R is degraded by the protoesome, and (5) β-arrestin mediates the activation of ERK from internalized “signalosomes.” (6) ERK then translocates to the nucleus and activates transcription.
(C) Krz mediates the ubiquitination and degradation of Drosophila Notch. (1) A Notch ligand on the Notch-ligand presenting cell binds Notch. (2) This event triggers the formation of a complex between Krz and the E3 ligase Dx. (3) Krz brings Dx to Notch and promotes Notch ubiquitination, (4) which leads to the Krz-dependent internalization of Notch, and (5) its subsequent degradation.