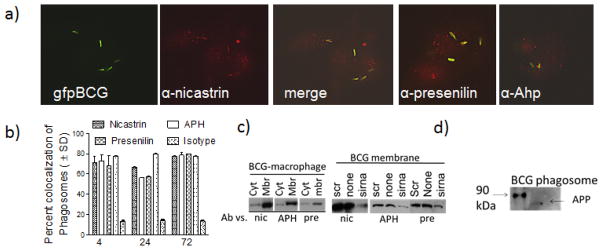
Figure 1. Macrophages infected with mycobacteria colocalize with γ-secretase components.
Antibodies to γ-secretase stain phagosomes with less cytoplasmic localization.(a)C57Bl/6 mouse bone marrow derived macrophages were infected with Mycobacterium bovis BCG vaccine strain tagged with gfp (gfp BCG) and stained with an antibody to nicastrin, APH or presenilin. Panel shows the three color panels for nicastrin and merged images for APH and presenilin, followed by Texas-red conjugates. b) Phagosomes colocalizing with γ-secretase components were scored using antibody stains and deconvolution microscopy (1 of 3 similar experiments, ± SD). Isotype antibody did not stain phagosomes and gfp BCG in dendritic cells showed a similar pattern (not shown). Membrane vs. cytosolic distribution of γ-secretase. (c) Treated or untreated BCG infected macrophages were fractionated into membrane and cytosol fractions and tested using antibodies and western blots. (Left) Untreated fractions:γ-secretase components are enriched in membranes. (Right) Treated fractions: siRNA knock-down reduces the levels of γ-secretase components in the membrane fractions, compared to knock down with scrambled sequence. (d) γ-secretase is active on the phagosomes. BCG phagosomes purified from treated or untreated macrophages were incubated in buffer to determine the cleavage of amyloid precursor protein into an 87 kDa APP product (arrow).