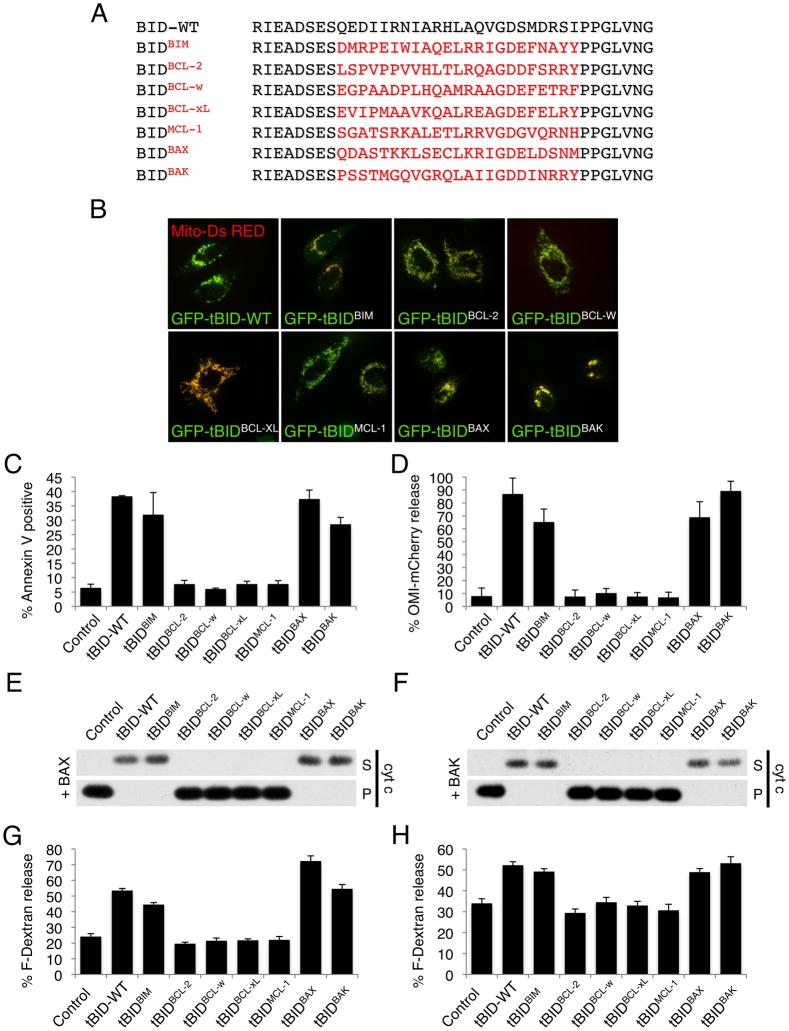
Figure 1. tBIDBAX-BH3 and tBIDBAK-BH3 directly activate BAX and BAK.
(A) Sequence alignment of the BH3 domains used to generate the tBIDBH3 chimeras. (B) HeLa cells were co-transfected with mitochondrial matrix targeted dsRed (mito-dsRed) and the indicated GFP-tBIDBH3 chimera in presence of Q-VD-OPH (20 μM) and imaged after 24 hr by live-cell confocal microscopy. Representative micrographs are shown. (C) HeLa cells were transfected with the indicated tBIDBH3 chimeras and apoptotic cell death was quantified after 24 hr by Annexin V staining and flow cytometry analysis. (D) HeLa cells stably expressing Omi-mCherry were transfected with tBIDBH3 chimeras and scored for mitochondrial versus cytosolic Omi-mCherry localization after 24 hr. (E) Mitochondria-enriched heavy membrane (MHM) fractions extracted from bak−/− C57BL/6 mouse liver were incubated with 10 nM purified recombinant BAX and IVTT (in vitro transcription and translation) from wheat germ extract expressing the indicated tBIDBH3 chimera for 1 hr at 37°C. Supernatant (S) and pellet (P) fractions were then separated by centrifugation and cytochrome c release was monitored by Western blot. (F) MHM extracted from WT C57BL/6 mouse liver were treated as in (E) in the absence of recombinant BAX. (G) Large Unilamelar Vesicles (LUV) containing Fuorescein-Dextran were incubated with IVTT of the indicated tBIDBH3 chimera in presence of 120 nM purified recombinant BAX. LUV permeabilization is shown as % release of Fluorescein-Dextran. (H) LUV were incubated as in (G) in presence of 500 nM purified recombinant BAK-ΔC (lacking the C-terminus). Error bars represent standard deviation from triplicate data.
(See also Figure S1)