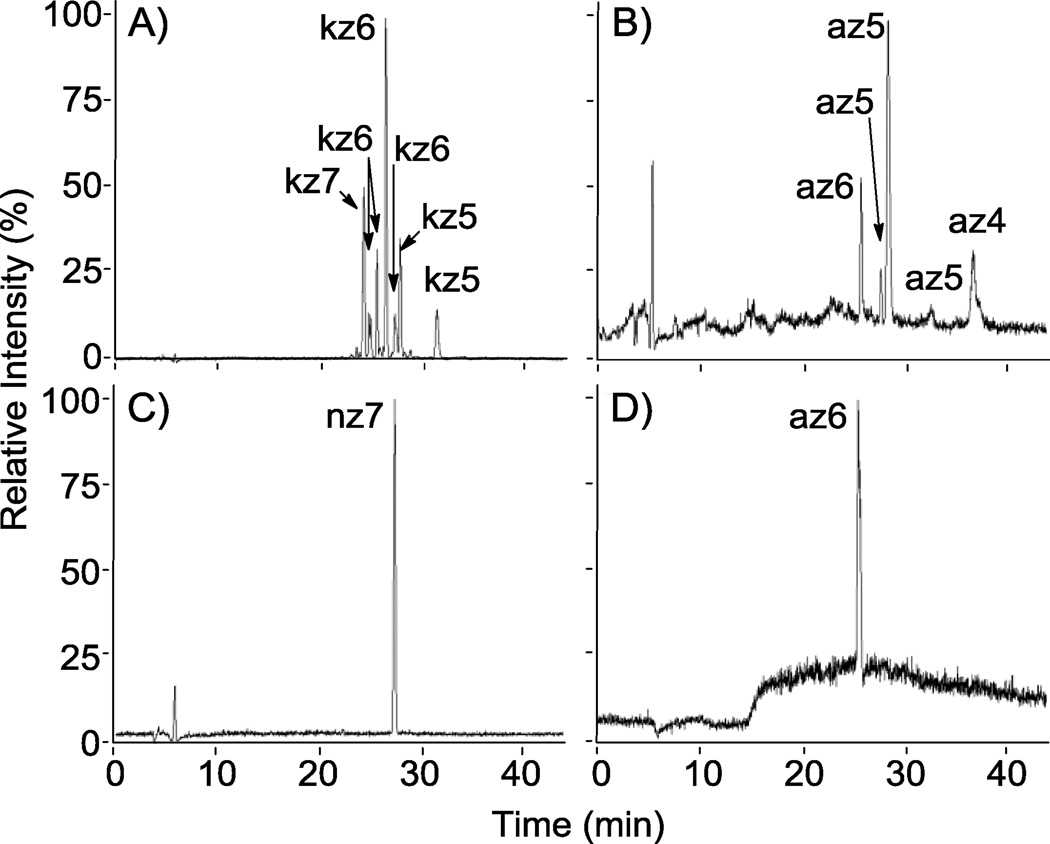
Figure 5.
RPIP-HPLC-MS total ion chromatograms (TICs) of reaction products from the sulfonation of N-cbz aminoglycosides. Shown are product mixtures from the sulfonation of kanamycin and apramycin with Pyr·SO3 (A and B, respectively) and the sulfonation of neomycin and apramycin with ClSO3H (C and D, respectively). A and B demonstrate separation of different sulfated states of N-cbz kanamycin (DS = 5–7) and N-cbz O-sulfonated apramycin (DS = 4–6), respectively. O-sulfonation with ClSO3H afforded persulfated products: (C) nz7 and (D) az6.