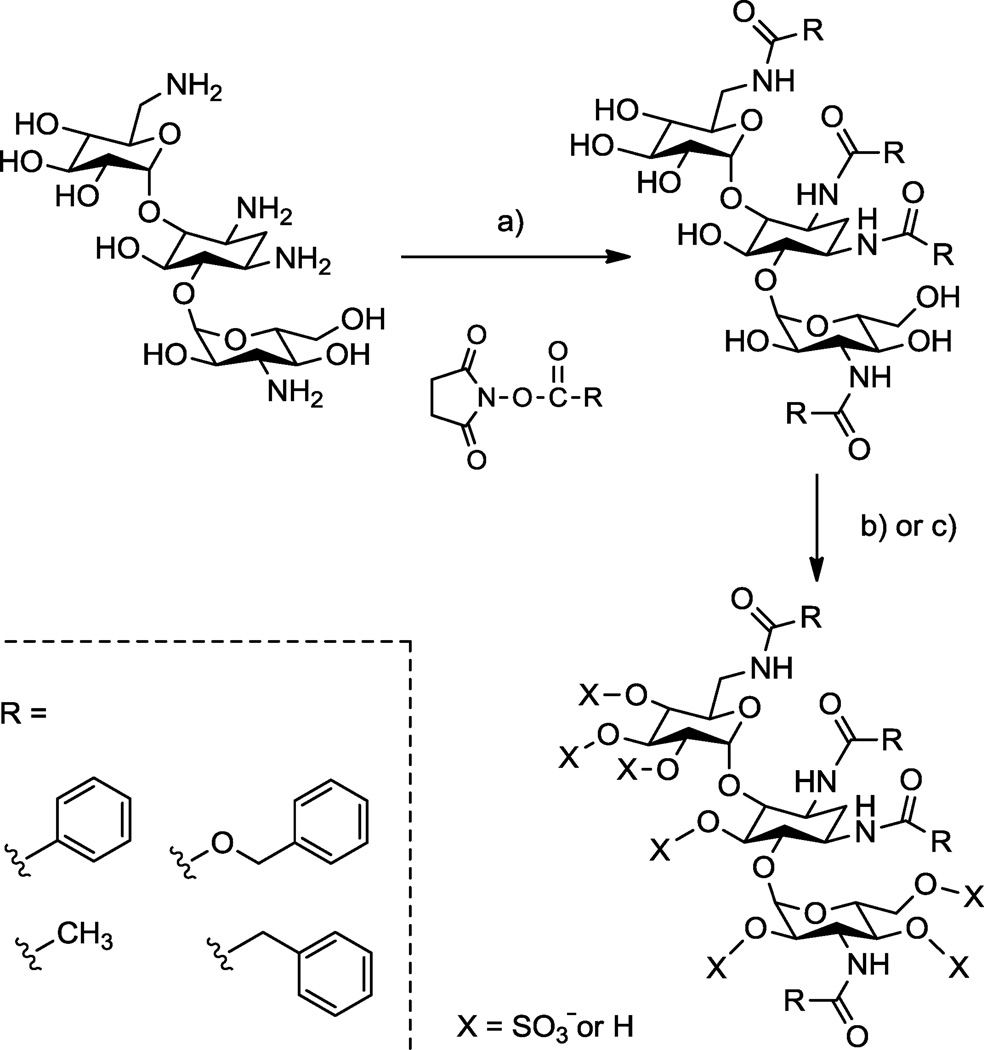
Scheme 1.
Representative synthesis showing concept for making N-aryl O-sulfonated aminoglycosides; shown is kanamycin. a) R-NHS, NaHCO3, H2O, 23 °C, 12–16 hr, 30–85%; b) i: Pyr·SO3, DMF, anhydrous pyr, 66 °C, 5–7 hr ii: H2O, 10 mM NaOH, 4 °C, 45 65%; c) i: ClSO3H, pyr, 57 °C, 4–6 hr, ii: H2O, NaHCO3, 35–95%.