Abstract
The beneficial effects of total parenteral nutrition (TPN) in improving the nutritional status of malnourished patients during hospital stays have been well established. However, recent randomized trials and meta-analyses have reported an increased rate of TPN-associated complications and mortality in critically ill patients. The increased risk of complications during TPN therapy has been linked to the development of hyperglycemia, especially during the first few days of TPN therapy. This retrospective study was conducted to determine whether the amount of dextrose from TPN in the 1st week in the intensive care unit (ICU) was related to the development of hyperglycemia and the clinical outcome. We included 88 non-diabetic critically ill patients who stayed in the medical ICU for more than two days. The subjects were 65 ± 16 years old, and the mean APACHE (Acute Physiology and Chronic Health Evaluation) II score upon admission was 20.9 ± 7.1. The subjects received 2.3 ± 1.4 g/kg/day of dextrose intravenously. We divided the subjects into two groups according to the mean blood glucose (BG) level during the 1st week of ICU stay: < 140 mg/dl vs ≥ 140 mg/dl. Baseline BG and the amount of dextrose delivered via TPN were significantly higher in the hyperglycemia group than those in the normoglycemia group. Mortality was higher in the hyperglycemia group than in the normoglycemia group (42.4% vs 12.8%, P = 0.008). The amount of dextrose from TPN was the only significant variable in the multiple linear regression analysis, which included age, APACHE II score, baseline blood glucose concentration and dextrose delivery via TPN as independent variables. We concluded that the amount of dextrose delivered via TPN might be associated with the development of hyperglycemia in critically ill patients without a history of diabetes mellitus. The amount of dextrose in TPN should be decided and adapted carefully to maintain blood glucose within the target range.
Keywords: Hyperglycemia, dextrose, parenteral nutrition, critically ill, non-diabetic
Introduction
Parenteral nutrition (PN) is an important means of providing nutrition to patients with a nonfunctional gastrointestinal tract, limited absorptive capacity, or other problems that prevent enteral feeding. Although PN has improved patient outcomes, recent randomized trials and meta-analyses have raised questions about its safety and the high rate of total parenteral nutrition (TPN)-associated complications and mortality in critically ill patients [1,2]. The current clinical guidelines for nutritional support in critically ill patients differ substantially by society. The Guidelines for the Nutrition Support Therapy in Critically Ill Patient by the American Society of Parenteral and Enteral Nutrition (ASPEN) and the Society of Critical Care Medicine (SCCM) [3] recommend early initiation of enteral nutrition but suggest that PN therapy be initiated after seven days for critically ill patients who are well nourished at baseline. In contrast, the guidelines of the European Society of Parenteral and Enteral Nutrition (ESPEN) recommended that practitioners consider initiating parenteral nutrition within two days of admission to the intensive care unit (ICU) for patients who cannot be adequately fed enterally [4].
The increased risk of TPN-associated complications can be related, among other factors, to the development of hyperglycemia [5,6]. TPN might cause hyperglycemia in patients with no history of diabetes mellitus [7]; hyperglycemia during TPN therapy can cause a higher mortality rate and prevalence of complications, especially infectious complications. However, there have been few studies to evaluate the effects of the amount of dextrose from TPN on the development of hyperglycemia. Therefore, we conducted this study to examine whether the amount of dextrose from TPN was associated with hyperglycemia and how it affects clinical outcomes in critically ill patients with no history of diabetes mellitus.
Subjects and Methods
Subjects
Between January 2009 and December 2009, 160 critically ill adult patients were admitted to the medical intensive care unit (ICU) of Severance Hospital, affiliated with Yonsei University College of Medicine in Seoul, Korea, where they stayed for more than two days. We analyzed the data of 88 patients after excluding 72 patients with a history of diagnosis and management of diabetes mellitus before ICU admission or treatment with steroid therapy during ICU care. We divided the subjects into two groups according to their mean blood glucose levels during the first week of ICU care: < 140 mg/dl as the normoglycemia group vs ≥ 140 mg/dl as the hyperglycemia group. The Institutional Review Board waived informed consent because this study was a retrospective chart review.
Methods
We retrospectively collected the patient data from the electric medical record. We recorded patients' general characteristics including age, gender, diagnosis, department of primary physician, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, length of stay and mortality in the ICU. We also collected data on TPN order and nutrition delivery, including the day of TPN initiation after ICU admission and carbohydrate delivery from TPN and enteral nutrition (EN), daily mean blood glucose (BG) level, and the insulin infusion rate during the first week in the ICU. Dextrose provided via the intravascular route included the total dextrose from TPN solution and 10% dextrose in water (D/W) solution, 20% D/W and 50% D/W solution (Choongwae Pharma Corp., Korea).
In our ICU, blood glucose levels were measured four times a day, every 6 hours. If the patients' BG level was measured at > 200 mg/dl twice, insulin infusion was started and BG level monitored every 2 hours until it reached the target range between 100 mg/dl and 140 mg/dl. We recorded four measurements of BG daily, even when the titration of insulin dose was checked more than four times. In the ICU, capillary blood glucose levels were measured using Accu-Chek Comfort Curative test strips (Roche Diagnostics GmbH, Germany) and a glucometer (Accu-Chek® Inform system, Roche Diagnostics GmbH, Germany).
Newly developed complications such as infection, acute renal failure and cardiac complications were recorded to analyze the relationship between hyperglycemia and the prevalence of complications. Infectious complications included infection of the urinary tract, respiratory system and bloodstream. Acute renal failure was defined as newly diagnosed renal failure requiring continuous renal replacement therapy in the ICU. Cardiac complications included myocardial infarction, arrhythmia and cardiogenic arrest.
Statistical analysis
All results were expressed as mean ± SD. We compared the two groups according to blood glucose level, using the chi-square test for categorical data and the student's t-test for continuous data after normalizing the variables that did not follow normal distribution: length of stay in ICU, duration of mechanical ventilation, overfeeding days during transitional feeding from TPN to EN, number of days of insulin use and carbohydrate delivery from EN. Multivariate analysis was conducted with stepwise multiple linear regression analysis. P < 0.05 was considered significant. Data were analyzed with PASW Statistics 18.0-August 2009 (SPSS in IBM, Armonk, NY).
Results
General characteristics
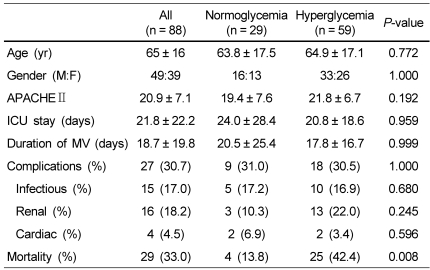
The general characteristics of the subjects are shown in Table 1. The subjects were 65 ± 16 years old, and 49 patients (55.7%) were male. APACHE II score within 24 hr after ICU admission was 20.9 ± 7.1. Length of stay in the ICU was 21.8 ± 22.2 days, and mortality in the ICU was 33%.
Table 1.
General characteristics of the normoglycemia and hyperglycemia groups
We divided the subjects into two groups according to the mean blood glucose level during the first week of ICU care: < 140 mg/dl, normoglycemia group vs ≥ 140 mg/dl, hyperglycemia group.
M:F, male:female; APACHE, Acute physiology and chronic health evaluation; ICU, Intensive care unit; MV, Mechanical ventilation
TPN was initiated 1.2 ± 0.6 days after ICU admission and provided for 14.4 ± 12.5 days. Five patients were not provided with TPN during their ICU stay, and TPN was not ordered for six patients during the first week of ICU stay. Average dextrose delivery from TPN and IV solution was 2.3 ± 1.4 g/kg/day. EN was provided to 75 patients within the first week of ICU admission and to 61 patients within three days of ICU admission. More calories than the estimated requirement were provided to 36 patients for 6.2 ± 6.0 days during the transitional process from TPN to EN.
Comparison between the two groups according to blood glucose levels
Fifty-nine patients (67%) were classified as the hyperglycemia group. There was no difference in patient characteristics between the groups (Table 1). Age, APACHE II score, duration of mechanical ventilation and length of ICU stay were identical between the two groups. Mortality in the ICU was significantly higher in the hyperglycemia group (42.4% vs 13.8%, P = 0.008), while the incidence of complications was not different between the two groups.
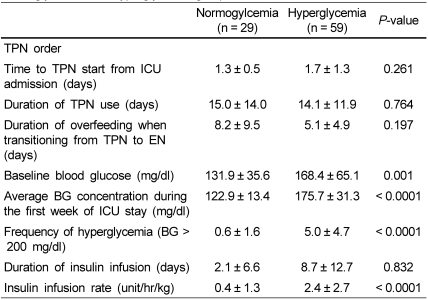
Patterns of PN order and BG levels are presented in Table 2. The time to initiation of TPN from ICU admission, duration of TPN use and overfeeding in the process of transitioning from TPN to EN were not significantly different between the two groups. Baseline glucose level, average BG concentration and frequency of hyperglycemia during the first week of ICU stay were significantly higher in the hyperglycemia group than in the normoglycemia group. While the duration of insulin infusion failed to show a statistically significant difference between the two groups, the average insulin infusion rate was significantly higher in the hyperglycemia group than in the normoglycemia group. (2.4 ± 2.7 units/hr/kg vs 0.4 ± 1.3 units/hr/kg, P < 0.0001)
Table 2.
TPN delivery, blood glucose concentration and insulin use in the normoglycemia and hyperglycemia groups
We divided the subjects into two groups according to the average blood glucose level during the first week of ICU care: < 140 mg/dl, normoglycemia group vs ≥ 140 mg/dl, hyperglycemia group.
BG, blood glucose; ICU, Intensive care unit; TPN, total parenteral nutrition; EN, Enteral nutrition
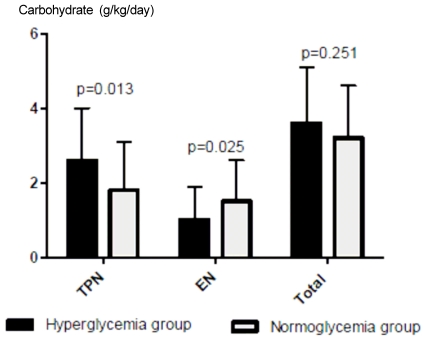
More dextrose was provided via TPN and other IV fluids in the hyperglycemia group (2.6 ± 1.4 g/kg/day vs 1.8 ± 1.3 g/kg/day, P = 0.013) (Fig. 1). Carbohydrate delivery via the enteral route was significantly less in the hyperglycemia group than in the normoglycemia group, and total carbohydrate delivery was not different between the two groups.
Fig. 1.
Amount of carbohydrate delivery via TPN and EN. We divided the subjects into two groups according to the mean blood glucose level during the first week of ICU care: < 140 mg/dl, normoglycemia group vs ≥ 140 mg/dl, hyperglycemia group. TPN, total parenteral nutrition; EN, enteral nutrition
We conducted multiple linear regression analysis including age, APACHE II score, baseline BG concentration and dextrose delivery via the parenteral route. Dextrose delivery from PN was found to be the only significant variable (Table 3). When dextrose delivery from TPN was increased by 1 g, BG concentration increased by 7.2 mg/dl (CI 1.29-13.31, P = 0.021).
Table 3.
Stepwise multiple regression analysis
Independent variables: Age, APACHE II (Acute Physiology and Chronic Health Evaluation II) score, Baseline blood glucose concentration, dextrose delivery via TPN
Discussion
We demonstrated that dextrose delivery from TPN and mortality were significantly higher in the hyperglycemia group during the 1st week of ICU care, which was similar to the results of previous studies [8,9]. Lin et al. [8] demonstrated that mortality was 2-5 times higher and the incidence of complications was 4-5.5 times higher in the group with BG ≥ 137 mg/dl than in the group with BG < 114 mg/dl. Cheung et al. [5] reported that mortality in patients with BG ≥ 9.1 mmol/l (164 mg/dl) was 11 times higher than that of patients with BG < 6.9 mmol/l (124 mg/dl), and this difference was more obvious in individuals without pre-diagnosed diabetes mellitus. Meanwhile, Pasquel et al. [9] reported that blood glucose before and within 24 h of starting TPN was associated with an increased risk of complications and mortality.
The target BG level for critically ill patients has been a controversial issue. Van den Berghe et al. [10,11] reported that intensive insulin therapy aiming for a BG level of less than 110 mg/dl could reduce the incidence of complications and mortality in surgical and medical intensive care units. Another multicenter study [12] suggested that BG ≤ 140-150 mg/dl was the target range to improve clinical outcomes and decrease the risk of lethal hypoglycemia. However, these studies did not consider delivery of dextrose (or carbohydrate) from PN or EN, which could be closely related to the development of hyperglycemia. Our data showed that the amount of dextrose delivered via TPN was significantly higher in the hyperglycemia group, and the amount of dextrose from TPN was a significant variable upon multiple linear regression analysis. It was recommended that no more than 4 mg/kg/min of dextrose from PN be provided to the critically ill and 7 mg/kg/min for stable patients in order to prevent complications including hyperglycemia [13]. For diabetes mellitus (DM) patients, TPN should be initiated with dextrose of ≤ 100-150 g/day and then increased to the goal rate after BG level is maintained within the target range [14]. Our subjects had not been diagnosed with DM before ICU admission, and the average amount of dextrose from TPN in the hyperglycemia group was 2.6 ± 1.4 g/kg, which was within the recommended range. However, the amount of carbohydrates from EN was smaller in the hyperglycemia group than in the normoglycemia group, and the total amount of carbohydrates from EN and TPN did not differ between the two groups. EN carbohydrate delivery was not found to be associated with BG levels upon multiple regression analysis when considering EN carbohydrate delivery instead of PN dextrose.
In this study, TPN was initiated within the first week of ICU care in 75 patients (85.2%), and 61 (81.3%) of these were provided with EN within three days, suggesting that many patients were prescribed TPN despite their ability to tolerate EN. One study reported that 30-40% of the patients who were administered TPN were inadequately ordered [15], and that inadequate use of TPN could be reduced to less than 10% by implementing proper guidelines, supervision and nutrition support team activity [16]. Therefore, complications associated with hyperglycemia might be reduced by implementing guidelines for proper PN initiation and monitoring. The proper timing for TPN initiation has not yet been decided. Current clinical guidelines for nutritional support in critically ill patients differ substantially across the continents [3,4], indicating the need for more studies. Our results support the ASPEN guidelines, which recommend early initiation of EN and delayed initiation of TPN for critically ill patients who are well-nourished at baseline.
We expected higher incidences of complications in the hyperglycemia group, but complication incidence was similar between the two groups. A higher rate of bloodstream infection was reported in the TPN treated patients [17]. A study on hematopoietic stem cell transplant recipients reported that a significantly greater number of TPN recipients required transfusion and delayed engraftment time [18]. Prospective studies will be needed to further elucidate the complications of TPN.
This study had some limitations. First, we compared the clinical outcomes on the basis of the BG concentration during the first week of ICU care. Although the mortality of the hyperglycemia group was higher than that of the normoglycemia group, we could not exclude the possibility of aggravation of the medical condition after the first week of ICU stay. However, Pasquel et al. [9] demonstrated that BG level prior to and within 24 h of PN initiation was associated with clinical outcomes rather than the average BG level during TPN therapy, emphasizing the importance of initial control of the BG level. It may be possible to overcome this limitation if we prospectively monitor the subjects' daily SOFA (sequential organ failure assessment) scores, which are used to track patients' status during their stay in an ICU [19]. Second, we considered the effect of carbohydrate on the BG level. Protein and fat, especially monounsaturated fatty acids and short chain fatty acids, can affect insulin sensitivity and/or gluconeogenesis [20-22]. Provision of protein and fat via TPN or EN might contribute to reducing the incidence of hyperglycemia by compromising the catabolic effect of body protein and body fat as the response to stress, which precluded further investigation.
In conclusion, our study showed that the amount of dextrose delivered via TPN might be associated with the development of hyperglycemia and poor clinical outcomes in critically ill patients without a history of diabetes mellitus and should be adapted carefully to maintain blood glucose within the target range.
References
- 1.Heyland DK, Montalvo M, MacDonald S, Keefe L, Su XY, Drover JW. Total parenteral nutrition in the surgical patient: a meta-analysis. Can J Surg. 2001;44:102–111. [PubMed] [Google Scholar]
- 2.der Voort PH, Feenstra RA, Bakker AJ, Heide L, Boerma EC, van der Horst IC. Intravenous glucose intake independently related to intensive care unit and hospital mortality: an argument for glucose toxicity in critically ill patients. Clin Endocrinol (Oxf) 2006;64:141–145. doi: 10.1111/j.1365-2265.2006.02437.x. [DOI] [PubMed] [Google Scholar]
- 3.McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G A.S.P.E.N. Board of Directors; American College of Critical Care Medicine; Society of Critical Care Medicine. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2009;33:277–316. doi: 10.1177/0148607109335234. [DOI] [PubMed] [Google Scholar]
- 4.Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, Griffiths R, Kreyman G, Leverve X, Pichard C ESPEN. ESPEN Guidelines on Parenteral Nutrition: intensive care. Clin Nutr. 2009;28:387–400. doi: 10.1016/j.clnu.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Cheung NW, Napier B, Zaccaria C, Fletcher JP. Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition. Diabetes Care. 2005;28:2367–2371. doi: 10.2337/diacare.28.10.2367. [DOI] [PubMed] [Google Scholar]
- 6.Heuer JG, Sharma GR, Zhang T, Ding C, Bailey DL, Stephens EJ, Holmes KC, Grubbs RL, Fynboe KA, Chen YF, Jakubowski JA. Effects of hyperglycemia and insulin therapy on outcome in a hyperglycemic septic model of critical illness. J Trauma. 2006;60:865–872. doi: 10.1097/01.ta.0000215565.29846.ab. [DOI] [PubMed] [Google Scholar]
- 7.Rosmarin DK, Wardlaw GM, Mirtallo J. Hyperglycemia associated with high, continuous infusion rates of total parenteral nutrition dextrose. Nutr Clin Pract. 1996;11:151–156. doi: 10.1177/0115426596011004151. [DOI] [PubMed] [Google Scholar]
- 8.Lin LY, Lin HC, Lee PC, Ma WY, Lin HD. Hyperglycemia correlates with outcomes in patients receiving total parenteral nutrition. Am J Med Sci. 2007;333:261–265. doi: 10.1097/MAJ.0b013e3180536b26. [DOI] [PubMed] [Google Scholar]
- 9.Pasquel FJ, Spiegelman R, McCauley M, Smiley D, Umpierrez D, Johnson R, Rhee M, Gatcliffe C, Lin E, Umpierrez E, Peng L, Umpierrez GE. Hyperglycemia during total parenteral nutrition: an important marker of poor outcome and mortality in hospitalized patients. Diabetes Care. 2010;33:739–741. doi: 10.2337/dc09-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 11.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 12.Van den Berghe G, Schetz M, Vlasselaers D, Hermans G, Wilmer A, Bouillon R, Mesotten D. Clinical review: Intensive insulin therapy in critically ill patients: NICE-SUGAR or Leuven blood glucose target? J Clin Endocrinol Metab. 2009;94:3163–3170. doi: 10.1210/jc.2009-0663. [DOI] [PubMed] [Google Scholar]
- 13.Sacks G, Mayhew S, Johnson D. Parenteral nutrition implementation and management. In: Merrit R, editor. The A.S.P.E.N. Nutrition Support Practice Manual. 2nd ed. Sliver Spring: American Society for Parenteral and Enteral Nutrition; 2005. pp. 108–117. [Google Scholar]
- 14.McMahon M, Manji N, Driscoll DF, Bistrian BR. Parenteral nutrition in patients with diabetes mellitus: theoretical and practical considerations. JPEN J Parenter Enteral Nutr. 1989;13:545–553. doi: 10.1177/0148607189013005545. [DOI] [PubMed] [Google Scholar]
- 15.Trujillo EB, Young LS, Chertow GM, Randall S, Clemons T, Jacobs DO, Robinson MK. Metabolic and monetary costs of avoidable parenteral nutrition use. JPEN J Parenter Enteral Nutr. 1999;23:109–113. doi: 10.1177/0148607199023002109. [DOI] [PubMed] [Google Scholar]
- 16.Saalwachter AR, Evans HL, Willcutts KF, O'Donnell KB, Radigan AE, McElearney ST, Smith RL, Chong TW, Schirmer BD, Pruett TL, Sawyer RG. A nutrition support team led by general surgeons decreases inappropriate use of total parenteral nutrition on a surgical service. Am Surg. 2004;70:1107–1111. [PubMed] [Google Scholar]
- 17.Dissanaike S, Shelton M, Warner K, O'Keefe GE. The risk for bloodstream infections is associated with increased parenteral caloric intake in patients receiving parenteral nutrition. Crit Care. 2007;11:R114. doi: 10.1186/cc6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheean PM, Freels SA, Helton WS, Braunschweig CA. Adverse clinical consequences of hyperglycemia from total parenteral nutrition exposure during hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:656–664. doi: 10.1016/j.bbmt.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Vincent JL, Moreno R. Clinical review: scoring systems in the critically ill. Crit Care. 2010;14:207. doi: 10.1186/cc8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin Y, Park S, Choue R. Comparison of time course changes in blood glucose, insulin and lipids between high carbohydrate and high fat meals in healthy young women. Nutr Res Pract. 2009;3:128–133. doi: 10.4162/nrp.2009.3.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokoyama J, Someya Y, Yoshihara R, Ishii H. Effects of high-monounsaturated fatty acid enteral formula versus high-carbohydrate enteral formula on plasma glucose concentration and insulin secretion in healthy individuals and diabetic patients. J Int Med Res. 2008;36:137–146. doi: 10.1177/147323000803600117. [DOI] [PubMed] [Google Scholar]
- 22.Pohl M, Mayr P, Mertl-Roetzer M, Lauster F, Lerch M, Eriksen J, Haslbeck M, Rahlfs VW. Glycaemic control in type II diabetic tube-fed patients with a new enteral formula low in carbohydrates and high in monounsaturated fatty acids: a randomised controlled trial. Eur J Clin Nutr. 2005;59:1221–1232. doi: 10.1038/sj.ejcn.1602232. [DOI] [PubMed] [Google Scholar]