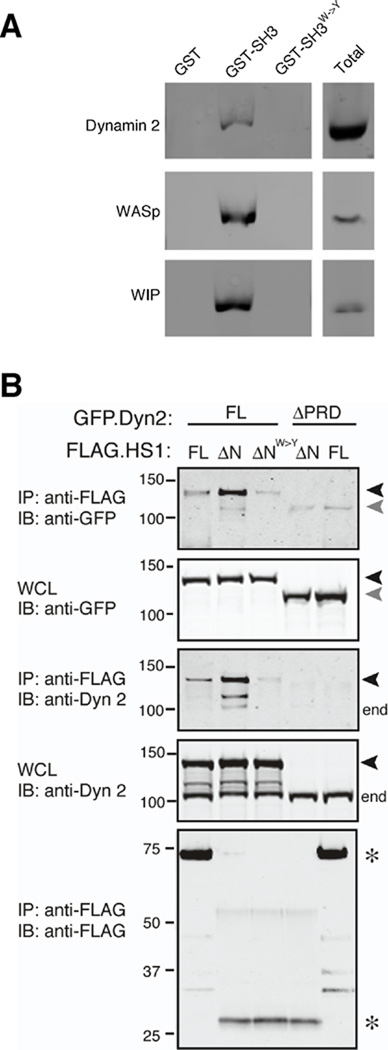
Fig. 6. The SH3 domain of HS1 interacts with the proline-rich domain of dynamin 2.
(A) GST alone, the SH3 domain of HS1 fused to GST, or the inactive SH3W->Y mutant fused to GST were immobilized on glutathione resin and incubated with DC lysates. Bound proteins were analyzed by SDS-PAGE and immunoblotting with anti-dynamin 2, anti-WASp or anti-WIP. Total, protein expression in whole cell lysates. (B) 293T cells were transiently transfected with full length GFP-tagged dynamin 2 (GFP.Dyn2 FL, black arrowheads) or a mutant lacking the proline rich domain (ΔPRD, gray arrowheads), together with FLAG-tagged HS1 (FLAG.HS1 FL), an N-terminal deletion mutant of HS1 lacking the actin regulatory region (ΔN), or the ΔN mutant bearing a point mutation in the SH3 domain (ΔNW->Y). Cells were lysed, immunoprecipitated with anti-FLAG, and western blots were probed as indicated. Note that the ΔPRD mutant is not detected by the anti-dynamin 2 (which was generated against this C-terminal region of dynamin 2), but is readily detectable in the WCL using anti-GFP antibody. end, endogenous dynamin 2; *, HS1 variants; WCL, whole cell lysate from which immunoprecipitation was performed.