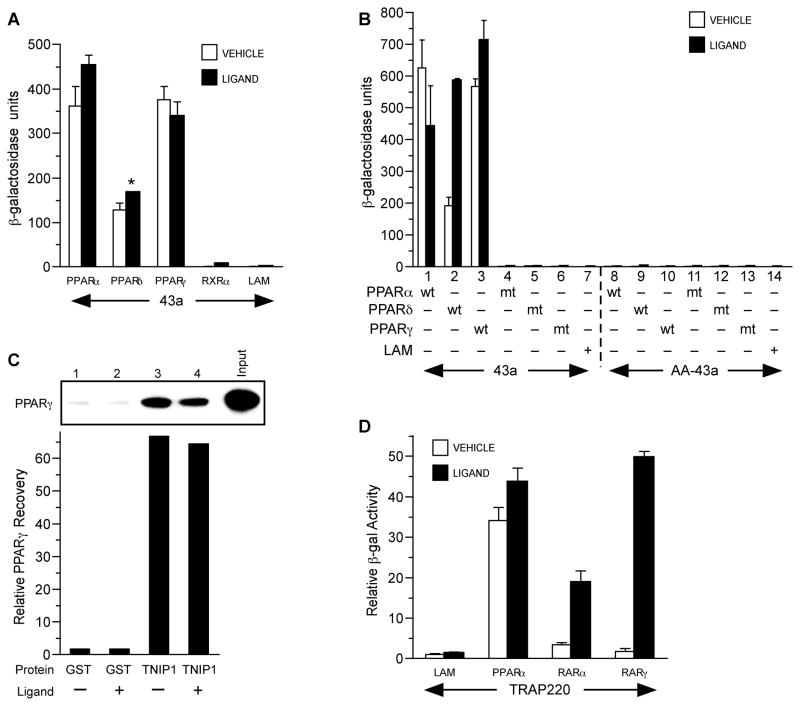
Fig. 2. PPARs interact with clone 43a and full-length TNIP1.
(A) PPAR interaction with 43a in yeast two-hybrid assays. Y187 yeast transformed with pGAD10-43a was mated to AH109 yeast transformed with pGBKT7-PPARs, RXRα, or lamin C as negative control (LAM). Vehicle, 0.1% DMSO. Ligands: PPARα and LAM, 10μM WY14,643; PPARδ, 3μM carbaprostacyclin; PPARγ, 10μM troglitazone; RXRα, 1μM 9-cis-RA.
(B) PPAR AF-2 and 43a NR box are required for interaction. Pairs of bars are vehicle or ligand treatment of cultures coexpressing wild-type (wt) or mutant (mt) receptors with wild-type (43a) or double alanine NR box mutant 43a (AA-43a). Pairs 1-3, wild-type (wt) receptor and wild-type 43a. Pairs 4-6, AF-2 helix 12 mutant (mt) for each PPAR and wild-type 43a. Pair 7, lamin C (LAM) and wild-type 43a as negative control. Pairs 8-10, wild-type (wt) receptor and NR box mutant 43a (LKKLL → LKKAA indicated as AA-43a). Pairs 11–13, helix 12 mutant (mt) receptor and 43a NR box mutant AA-43a. Pair 14, LAM and NR box mutant AA-43a as negative control. Culture conditions, vehicle and ligand treatment as in A.
(C) PPARγ interacts in vitro with GST-TNIP1 full-length protein. Graph is from phosphor-imager arbitrary units of dried gel. GST, glutathionine S transferase. TNIP1, fusion protein of GST and full-length TNIP1. Graph bars correspond to gel lanes 1-4. Input is 20% of labeled PPARγ used for pull-down. Lane 1, PPARγ incubated with GST and vehicle; lane 2, PPARγ with GST and ligand; lane 3, PPARγ with GST-TNIP1 fusion and vehicle; lane 4, PPARγ with GST-TNIP1 fusion and ligand. Vehicle, DMSO, 0.1%. Ligand, 1μM troglitazone.
(D) Ligand effect on TRAP220 interaction with PPARα versus RARα and γ interaction is receptor-specific. Two-hybrid conditions and PPAR ligands as in A, RARα and RARγ ligand is 1μM retinoic acid. Reporter β-galactosidase activity is presented as fold relative to control protein lamin C (LAM) and vehicle (0.1% DMSO) set to 1 as per Zhu et al [33].