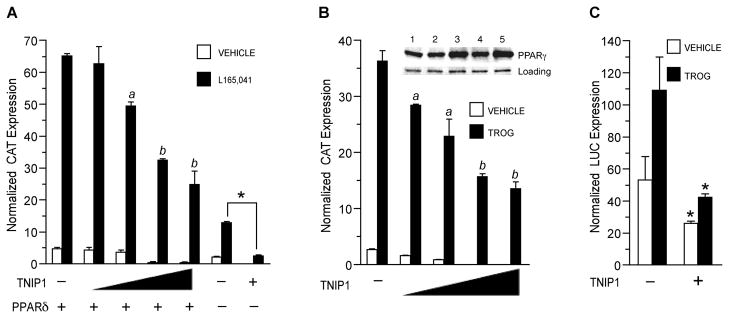
Fig. 5. PPAR activity is reduced by expression of full-length TNIP1.
(A) TNIP1 represses PPAR™ transactivation. COS-7 cells transfected with PPRE reporter p3xZ-CAT, pSG5-PPAR™, and pCMV-HA-TNIP1 at 0.1, 0.5, 1.0, or 1.5μg (indicated by triangle). Total plasmid copy number was kept constant by addition of empty pCMV-HA. Transfection with 1.5μg pCMV-HA-TNIP1in the absence of pSG5-PPARδ (−) is represented in the last 2 bar sets. PPARδ ligand; 1μM L165,041. Vehicle, 0.1% DMSO. For the ligand-treated set, a, b, are different from each other and cultures receiving pCMV-HA empty vector instead of pCMV-HA-TNIP1 (p < 0.05, one-way ANOVA, Tukey’s test). Asterisk, significant difference between ligand-treated cultures from endogenous receptor activity (no PPARδ transfected) without and with TNIP1 expression (t-test, p < 0.001). The PPAR heterodimer partner RXRα was included in the transfections as described [18].
(B) TNIP1 represses PPARγ transactivation. COS-7 cells transfected with PPRE reporter and TNIP1 constructs as in (A). TROG, 5μM troglitazone. Vehicle, 0.1% DMSO. Statistical comparison (one-way ANOVA, Tukey’s test; p < 0.05) of ligand-treated cultures: a, b are different from each other and cultures receiving pCMV-HA empty vector instead of pCMV-HA-TNIP1. Inset, western detection of endogenous PPARγ and loading control from lysates of TNIP1-transfected, ligand-treated cells used in CAT assay. Lane 1: 1.5μg pCMV-HA-empty vector. Lanes 2 – 5: 0.1, 0.5, 1.0, or 1.5μg pCMV-HA-TNIP1.
(C) TNIP1 represses endogenous PPARγ transactivation in differentiating mouse 3T3-L1 cells. 3T3-L1 cells were transfected overnight with PPRE reporter and pCMV-HA-empty vector (−) or pCMV-HA-TNIP1 (+) and then cultured in differentiation induction media for 48 h with 5μM troglitazone (TROG) or vehicle, 0.1% DMSO. Asterisk, (t-test, p < 0.02) reporter activity in cultures receiving TNIP1 were reduced from those receiving empty vector.