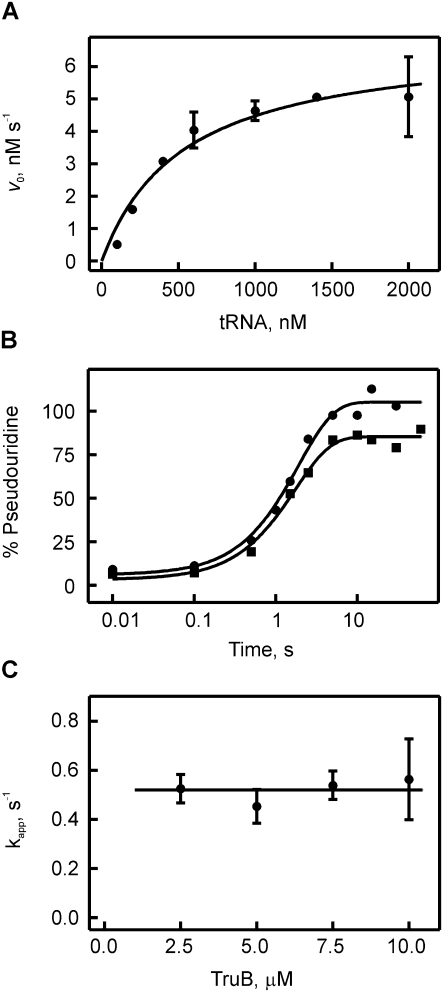
FIGURE 3.
Michaelis-Menten and quench-flow analysis of pseudouridine formation by TruB. (A) Michaelis-Menten experiment of TruB. Ten nM of enzyme was mixed with increasing concentrations of [3H]-labeled tRNAPhe, and pseudouridine formation was detected using the tritium release assay. The dependence of the initial rates v0 on the tRNA concentration was fitted to the Michaelis-Menten equation yielding a Michaelis constant, KM, of 550 ± 150 nM and a catalytic constant, kcat, of 0.7 ± 0.2 sec−1 for TruB. (B) Time courses of pseudouridine formation by TruB under single-round, pre-steady-state conditions. [3H]-labeled tRNAPhe (1 μM final concentration) was rapidly mixed with TruB (circles: 2.5 μM, squares: 10 μM final concentration) in a quench-flow apparatus. The percentage of pseudouridine formed at a certain time point was determined using the modified tritium release assay. The apparent rate of pseudouridine formation for each TruB concentration was determined by fitting the time courses to a one-exponential function (smooth lines). (C) Dependence of the apparent rate of pseudouridine formation under single-round conditions on the enzyme concentration. The average apparent rate is 0.5 ± 0.2 sec−1 for TruB, as indicated by the horizontal line.