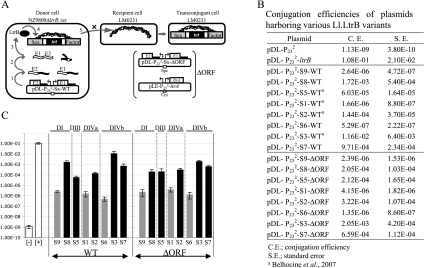
FIGURE 2.
Ll.LtrB trans-splicing/conjugation assay and SF conjugation rates. (A) The trans-splicing efficiency of Ll.LtrB is monitored by the conjugation rate of the SF between two L. lactis strains. The donor cell harbors the chromosomal SF with a defective relaxase gene (NZ9800ΔltrB::tet) while the recipient cell lacks the SF (LM0231). The Ll.LtrB intron, along with portions of its exons, was replaced in the chromosome of NZ9800 by a tetracycline resistance marker (tet), which prevents expression of the relaxase (LtrB). The LtrB deficient strain is complemented by providing the interrupted ltrB gene from a plasmid. Following transcription of the gene pieces (step 1), intron fragments assemble, Ll.LtrB trans-splices, and the flanking exons are ligated (step 2). Mature mRNA is translated and leads to expression of the relaxase enzyme (LtrB) (step 3), which recognizes the SF origin of transfer (oriT) (step 4) and initiates its transfer from a donor to a recipient cell by conjugation (step 5). Transfer efficiency of conjugative elements between L. lactis strains was previously shown to be directly proportional to Ll.LtrB splicing from the relaxase transcript (Klein et al. 2004; Belhocine et al. 2007). In a second version of the assay, the pLE-LtrA plasmid was introduced in various L. lactis strains (NZ9800ΔltrB::tet/pDL-P232-Sx-ΔORF) to provide LtrA in trans (between brackets). Ll.LtrB group II intron, black line; exon 1 and 2, E1 and E2; P23 promoter, bent arrows; SF, gray; intron RNA fragments, black wavy lines; RNA exons, white wavy lines; LtrB, black oval; spectinomycin resistance marker, Spc; chloramphenicol resistance marker, Cm; L. lactis chromosome, scribble. (B,C) SF conjugation efficiency in the presence of the ltrB gene interrupted by various Ll.LtrB fragmented introns (S1–S9 WT and ΔORF). Gray bars, bipartite introns with no potential base-pairing interactions; black bars, bipartite introns with potential base-pairing interactions; white bars, negative ([−], pDL-P232, empty vector) and positive ([+], pDL-P232-ltrB, full length Ll.LtrB) controls.