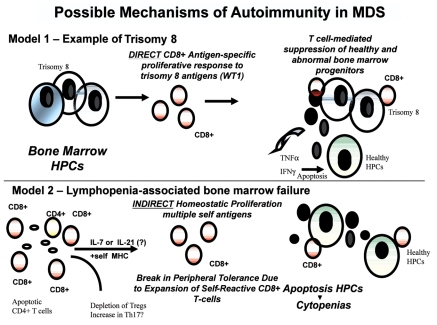
Figure 1.
Molecular model of T-cell pathogenesis in MDS. Described in this review are the supporting data that link T-cell abnormalities to multiple abnormal events that include apoptotic response and the presence of inflammation in the bone marrow microenvironment. The mechanism controlling the expansion of self-reactive CD8+ T-cells appears to be two fold described by two models (DIRECT and INDIRECT); 1) there may be direct activation of CD8+ T cells in response to abnormally expressed bone marrow antigens as exemplified by patients with trisomy 8, and 2) there may be expansion of destructive CD8 T cells due to homeostatic cytokines of the IL-2Rβγcommon cytokines which include IL-7, IL-2, IL-15, and IL-21. From previous results, lymphopenia-associated autoimmunity has been linked to antigen-specific CD8+ T cells with variable self- antigen reactivity. Survival advantage and clonal expansion dominates from CD8+ self-reactive T cells that respond to antigen in the context of self-MHC class I bypassing peripheral tolerance mechanisms. Results suggest that self-reactive CD8+ T-cells are involved in the suppression of bone marrow progenitors through direct cytotoxicity of the MDS clones such as trisomy 8+ HPCs or the release of cytokines such as TNFα and IFNγ. There is also evidence that depletion of CD4+ regulatory T cells (Tregs) and accumulation of IL-17-secreting CD4+ T cells (Th17) may be imbalanced. Evidence supports the role of these T-cell abnormalities in cytopenias and increased apoptosis observed in lower-risk MDS. HPC=Hematopoietic Progenitor Cells, WT1=Wilms' tumor 1 antigen.