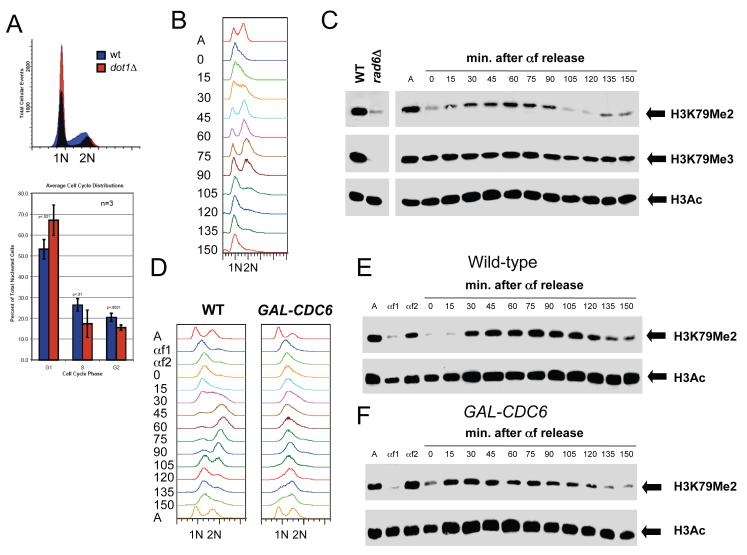
Figure 3. Cell cycle regulation of H3K79 dimethylation.
(A) Cells lacking DOT1 accumulate in G1. Wild-type and dot1Δ cells were grown to mid-log phase at 30°C in YPD and DNA content was analyzed by flow cytometry. The percentage of cells in G1 with 1N DNA content, S phase with intermediate DNA content and G2/M with 2N DNA content was quantitated using 3 independent samples. Error bars represent standard deviation of the mean. (B-C) H3K79 dimethylation increases during S phase. Wild-type cells (SLJ001) were arrested in G1 with α-factor at 30°C for 3 hr. They were released into fresh YPD at 30°C, and α-factor was added back when small buds appeared, to prevent cells from entering the next cell cycle. Samples were taken at the indicated times following release from α-factor; asynchronous cells (A) were also collected. (B) DNA content was analyzed by flow cytometry to estimate cell cycle position. Entry into S phase begins around 15-30 min. based on the shift of the 1N DNA peak, while cells have entered G2/M approximately 45-60 min. after α-factor release. By 90 min., cells have exited M phase and re-arrested in G1. (C) Cell cycle-dependent modification of H3 was analyzed by immunoblotting with anti-acetyl-H3 and anti-H3K79me2 and anti-H3K79me3 antibodies. Equal protein concentrations were loaded in all lanes as judged by total H3 levels (data not shown). (D-F) DNA replication is not required for cell cycle oscillations in H3K79 dimethylation. Wild-type and GAL-CDC6 cells were grown in YEP containing 2% raffinose and 4% galactose at 30°C then arrested in G1 with α-factor (αf1). Cells were released from G1 into YEP containing 2% raffinose and 4% galactose to allow CDC6 expression to initiate DNA replication. After 20 min., cells were transferred into YPD to repress GAL-CDC6 and α-factor was added at 45 min. to arrest cells in G1 (αf2). Cells were then released from G1 in glucose-containing media to analyze cell cycle progression in cells lacking Cdc6 protein. α-factor was added back when small buds appeared, to prevent cells from entering the next cell cycle. Samples were taken at the indicated times following release from the second α-factor release; asynchronous cells (A) were also collected. (D) DNA content was analyzed by flow cytometry. While wild-type cells replicate DNA (30 min.), progress through mitosis and rearrest in G1, similar to our results in (B), cells lacking Cdc6 protein do not undergo DNA replication. Peak drift in this sample is likely due to mitochondrial DNA since cells continue to increase in size. Cell cycle-dependent modification of H3 was analyzed by immunoblotting with anti-H3K79me2 and anti-acetyl-H3 antibodies in wild-type (E) and GAL-CDC6 (F) cells. Equal protein concentrations were loaded in all lanes as judged by total H3 levels (data not shown).