Figure 2.
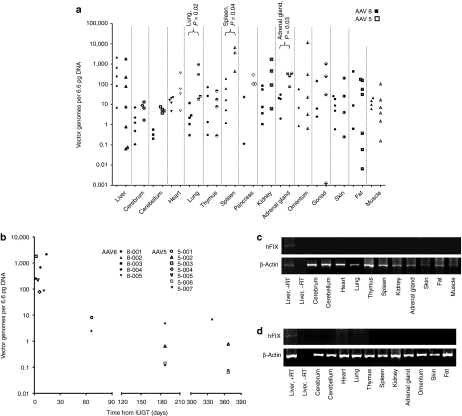
Other outcomes in treated offspring. Biodistribution of the vector of all animals (AAV8, n = 5; AAV5, n = 7) taken at necropsy or at 12 months survival surgery (5-002, 5-006, 5-007) showed comparable liver transduction with either vector (a), while AAV5 treated animals showed higher vector load in the lung, spleen, and adrenal glands (P < 0.03, nonparametric Mann–Whitney U test). Among the AAV8-treated animals there was higher VCN in the liver compared with the cerebrum, cerebellum, heart, and lung (P ≤ 0.03). Within the AAV5 group there was higher VCN in the spleen compared with the liver, skin, fat, and muscle (P ≤ 0.03). Hepatic vector biodistribution as a function of time from IUGT (b). Vector copy number in the liver decreased over four-log fold from birth to 12 months after IUGT. Delivery of either scAAV2/8-LP1-FIXco or scAAV2/5-LP1-FIXco resulted in liver-specific transgene expression as shown by RT-PCR (c,d). Shown below are representative data from offspring injected with AAV8 (at 11 months, c) and AAV5 (at 12 months, d). Gel images were captured with the AlphaDigiDoc gel documentation system (Alpha Innotech/Cell Biosciences, Santa Clara, California), AlphaEase FC/AlphaDigiDoc 1200 software and a 4 megapixel C4000 Zoom digital camera providing three times optical zoom (Olympus). AAV, adeno-associated viral vector; hFIX, human Factor IX; IUGT, intrauterine gene transfer; RT-PCR; reverse transcription PCR; VCN, vector copy number.