Abstract
Background
Antibodies to blood-stage Plasmodium falciparum antigens have been associated with protection against clinical malaria in some studies but not others. Many of these studies have not assessed whether high-titer antibodies are associated with protection and have not adjusted for differences in malaria exposure.
Methods
The presence of high-titer antibodies to apical membrane antigen-1 (AMA-1), erythrocyte binding antigen-175 (EBA-175) and merozoite surface protein-119 (MSP-119) was assessed in 87 children living in a malaria holoendemic area of Kenya. The children were prospectively assessed during one year for clinical malaria.
Results
In unadjusted analyses, high-titer antibodies to MSP-119, but not EBA-175 or AMA-1, were associated with protection from clinical malaria. However, after adjustment for exposure, only high-titer antibodies to EBA-175 were associated with protection from clinical malaria (hazard ratio (HR), 0.48, 95% confidence interval (CI) 0.24, 0.95, P=0.03), and with reduced episodes of clinical malaria (incidence rate ratio, 0.50, 95 % CI, 0.31, 0.81, P=0.005). A trend toward increased protection from clinical malaria in children was seen with antibodies to both EBA-175 and MSP-119 (HR, 0.26, 95% CI 0.03, 1.94, P=0.18)
Conclusions
High-titer antibodies to EBA-175 are associated with protection from clinical malaria in children in a malaria holoendemic area of Kenya. Accurate estimates of antibody-associated protection from clinical malaria require adjustment for malaria exposure.
Keywords: malaria, EBA-175, children, holoendemic
Introduction
Malaria causes more than 175 million clinical cases of infection and results in death for more than 710,000 children in sub-Saharan Africa each year [1]. The importance of humoral immunity in protection from clinical malaria has been demonstrated through studies of passive antibody transfer from semi-immune adults to children with resulting reduction in peripheral blood parasitemia and alleviation of clinical symptoms [2, 3]. Furthermore, non-sterile clinical immunity can be obtained by individuals living in endemic zones and is attributed to repeated exposure and subsequent immunologic response [4]; this observation suggests that a malaria vaccine, particularly an “anti-disease” vaccine, may be feasible. The morbidity and mortality associated with malarial disease occurs when Plasmodium falciparum is in the blood stage. This provides the rationale for pursuit of a blood stage vaccine [5]. Many recent efforts are focused on multiple antigen vaccines that will induce a broad repertoire of immune responses against the parasite.
We previously assessed the relationship of antibodies to the blood-stage vaccine candidate antigens apical membrane antigen-1 (AMA-1), erythrocyte binding antigen-175 (EBA-175) and merozoite surface protein-119 (MSP-119) and protection from blood-stage P. falciparum infection in adults in the malaria holoendemic area of Kanyawegi, Kenya. In that study, a trend toward a decreased risk of blood-stage infection was seen in adults with antibodies to AMA-1 but not MSP-119 or EBA-175, but we were not able to assess the correlation of these antibodies with protection from disease as none of these semi-immune adults developed clinical disease. Prior studies have presented conflicting results about the association of antibodies to AMA-1 [6–9], MSP-119 [10–14] and EBA-175 [15, 16] with protection from disease. In the present study, we assessed the relationship between antibodies to AMA-1, EBA-175 and MSP-119, separately and together, with protection from clinical malaria in children in the same malaria holoendemic area where we conducted our studies in adults.
Materials and methods
Study site and participants
The study was conducted in the Kanyawegi region of Nyanza Province, Kenya beginning in August, 2001 through July 2002 [17]. Kanyawegi is located in an area holoendemic for malaria with a population of approximately 3,500 individuals. All study participants were between the ages of 3 months and 8 years and were permanent residents. Exclusion criteria included acute or chronic illness, current symptoms of malaria, and use of anti-malaria drugs within the previous two weeks. Study participants were recruited randomly from all seven villages that comprised the study site. Eighty-seven children were recruited by written informed consent that was obtained from the parents or guardians of all participants. Ethical approval for the study was granted by the Kenya Medical Research Institute (KEMRI) Ethical Review Committee and the Institutional Review Board for Human Studies at the University Hospitals of Cleveland (Cleveland, OH) and Case Western Reserve University (Cleveland). Study participants received free medical care for malaria but did not receive other forms of compensation.
Procedures
Approximately 0.5–1 mL of blood was collected at the beginning of the study. Samples were centrifuged, and plasma was removed and stored at −80°C for antibody testing. Ten μL of blood was obtained to measure the hemoglobin concentration. Malaria infection was diagnosed by microscopic inspection of thick and thin blood smears. Blood smears were stained with Giemsa stain, and slides were examined by two experienced microscopists. The microscopists were blind to the study protocol and read each slide twice. A smear was deemed negative when no parasites were observed after counting microscopic fields that included at least 200 leukocytes. Density of parasitemia was expressed as the number of asexual P. falciparum organisms per microliter of blood, assuming a leukocyte count of 8,000 per μl. Antimalarial treatment was not given at the time of enrollment into the study as Kenyan national policy was not to treat children with asymptomatic parasitemia. As recommended by the Kenya Ministry of Health all enrolled children who developed clinical malaria during the 52- week follow-up period were given antimalarial therapy according to the national guidelines (at that time, sulfadoxine-pyrimethamine, or amodiaquine or quinine in children allergic to sulfadoxine-pyrimethamine).
Active surveillance for clinical malaria was conducted over a 52-week period after enrollment. Parents of children were told to contact their village-based field assistant if their child displayed symptoms of malaria (i.e. fever or chills). Village-based field assistants made weekly visits to study participant homes to assess whether any participant had fever or chills in the previous week or at the time of visit. Children with fever or chills were seen by a clinical officer and treated with antimalarial drugs if they had P. falciparum on a blood smear. An episode of clinical malaria was defined as self-report of fever or chills or an axillary temperature of >37.5°C, plus asexual-stage P. falciparum on a blood smear. A second end point of high-density parasitemia was defined as fever or chills plus an asexual-stage P. falciparum density of ≥4000 parasites/μL[18].
Recombinant antigens were used to test for the presence of antibodies to AMA-1, EBA-175, and MSP-119. Recombinant AMA-1 (ectodomain, nonglycosylated) and EBA-175 (region II, nonglycosylated) were expressed in Pichia pastoris and provided by one of the authors (DLN, National Institutes of Health). Recombinant MSP-119 protein corresponding to the E-KNG variant was expressed in Saccharomyces cerevisiae [19] and provided by the Malaria Research and Reference Reagent Resource Center (Manassas, VA). In previous testing in this area, IgG antibodies from adult sera most frequently recognized the E-KNG variant [20].
Total IgG and IgG subclass concentrations of antibodies were measured using enzyme-linked immunosorbent assay (ELISA), as described elsewhere [20]. All children were tested for antibodies to AMA-1 and EBA-175. Seventy-two of the 87 children with adequate remaining serum sample were tested for antibodies to MSP-119. Samples were tested in duplicate and a mean OD value was used in final analysis. Antibodies were expressed in arbitrary units (AU) and calculated by dividing the ODs for samples from study participants by the mean OD +3 standard deviations (SDs) for samples from 40 North Americans never exposed to malaria. Sera from nine North American controls with OD values representative of the 40 North Americans without malaria exposure were used on each plate. An OD value ≥1.0 AU was considered to be positive. Subclass analysis was done on a subset of 35 samples with adequate plasma for the additional subclass testing.
Statistical analysis
All statistical analysis was done using Stata 10.0 software (Stata Corporation, College Station, TX). The primary exposure variable was set before analysis as high-titer IgG antibodies (>2AU) to any of the blood-stage antigens tested (AMA-1, EBA-175, or MSP-119), based on our previous studies of association of antibody concentrations to time to infection in adults[20].
Correlations between continuous variables (e.g., concentrations of antibodies to different antigens) were assessed by Spearman rank correlation. Associations between categorical variables (e.g., presence of high concentrations of antibodies to specific antigens and clinical malaria) were assessed by chi-square analysis. Kaplan-Meier survival analysis and the log rank test were used to compare the times to development of P. falciparum parasitemia in study participants with and those without high concentrations of IgG antibodies to blood stage antigens. Cox proportional hazards regression modeling was used to assess the risk of the first symptomatic malaria episode. The cumulative incidence of clinical malaria episodes was assessed with negative binomial regression analysis, in which all malaria episodes during the 52-week period were recorded. Village, age, bed net use, and malaria infection status at the time of study enrollment were adjusted for in all final models.
Results
Study population characteristics
Eighty-seven children below the age of 8 years old were enrolled in the study (range 0.56 – 7.95 years; mean 5.22 years, standard deviation (SD) 1.72 years). At the time of enrollment 78 percent of the children (68/87) tested P. falciparum positive but all were asymptomatic. Only three children lived in households where bednets were used (3.4%). Three children moved out of the study area (at weeks 6, 17, and 33 respectively). Data were censored in Cox regression for these children on the dates at which they moved out.
Frequencies of high-titer IgG and IgG subclass antibodies to blood stage antigens
IgG antibody concentrations and the frequencies of children with high-titer antibodies (antibody concentrations >2 AU) are shown in Table 1. The majority of children had high-titer antibodies to AMA-1 (63%) and EBA-175 (53%), but high-titer antibodies to MSP-119 (26%) were less frequent. IgG subclass testing revealed that almost all high-titer IgG subclass antibodies to the three antigens were either IgG1 or IgG3 (Table 2). IgG2 and IgG4 subclass antibodies were absent except for IgG2 to EBA-175 (11.43%) and IgG4 to MSP-119 (2.8%).
TABLE 1.
Median IgG concentrations and frequency of high-titer IgG antibodies to AMA-1, EBA-175, and MSP-119 in Kenyan children <8 years of age.
| Antigen | Median concentration, AU* (range) | AU>2, (%) |
|---|---|---|
| AMA-1 | 3.84 (.02–17.73) | 55/87 (63.2) |
| EBA-175 | 2.68 (.04–77.61) | 46/87 (52.9) |
| MSP-119 | 1.31 (.03–14.63) | 19/72 (26.4) |
AU = Arbitrary Unit (see Methods for description of AU calculation).
TABLE 2.
Frequencies of high-titer (AU>2) IgG subclass antibodies to AMA-1, EBA-175, and MSP-119 in 35 Kenyan children
| Antigen | IgG1 (%) | IgG2 (%) | IgG3 (%) | IgG4 (%) |
|---|---|---|---|---|
| AMA-1 | 45.71 | 0 | 51.43 | 0 |
| EBA-175 | 71.43 | 11.43 | 74.29 | 0 |
| MSP-119 | 25.71 | 0 | 77.14 | 2.86 |
High-titer IgG antibodies and protection from clinical malaria
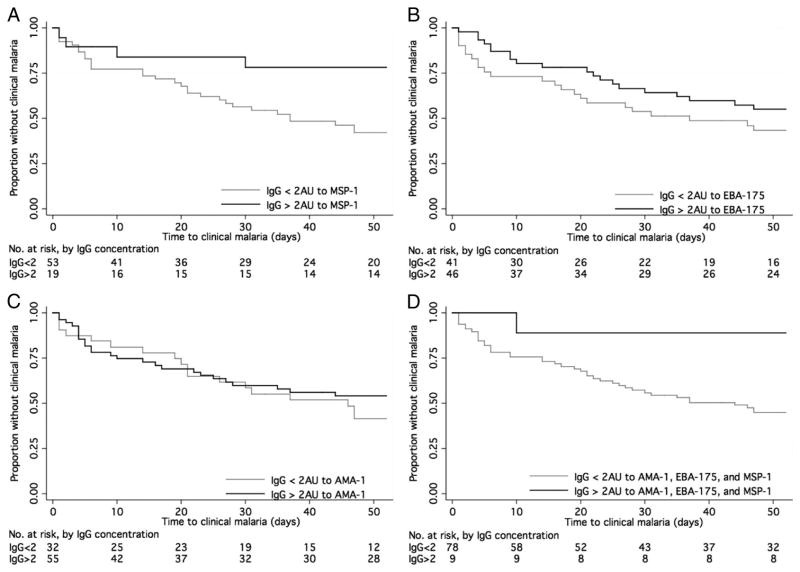
Kaplan-Meier analysis revealed an association with protection from clinical malaria for high-titer antibodies to MSP-119 (Figure 1A, P=0.02), and a weak trend toward protection for high-titer antibodies to EBA-175 (Figure 1B, P=0.19), but no association with protection with high-titer antibodies to AMA-1 (Figure 1C, P=0.45). A strong association with protection was seen in children with high-titer antibodies to all three antigens, as only one of the nine children with high-titer antibodies to all three antigens developed clinical malaria during the 52-week follow-up period (Figure 1D, P=0.03).
Figure 1.
Time to first clinical malaria episode in children with high concentrations of IgG antibodies to A. merozoite surface protein-119 (MSP-119); B. erythrocyte binding antigen-175 (EBA-175); C. apical membrane antigen-1 (AMA-1); D. AMA-1, EBA-175 and MSP-119.
Although the site of Kanyawegi comprises an area of <12 km2, considerable variation was seen in risk of malaria between the six site villages, notably a significantly increased risk in one of the six villages (village 3, hazard ratio (HR) 5.06, 95% confidence interval (CI) 1.84, 13.92, P=0.002). For this reason, village was added to the regression model as a surrogate for exposure. In the final Cox regression model, adjusting for age, exposure (village), bednet use, and presence of P. falciparum parasitemia at the time of enrollment, children with antibodies to MSP-119 still had a lower risk of clinical malaria (HR 0.46, 95% CI 0.13, 1.57, P=0.21) and lower incidence of clinical malaria (incidence rate ratio (IRR) 0.50, 95% CI 0.18, 1.31, P=0.16), but neither was statistically significant. In contrast, high-titer antibodies to EBA-175 were associated with a significantly lower risk of clinical malaria (HR 0.48, 95% CI 0.24, 0.95 P=0.03), and incidence of clinical malaria (IRR 0.50, 95 % CI, 0.31, 0.81, P=0.005). Antibodies to AMA-1 did not show a significant association with protection (Table 3).
TABLE 3.
Village adjusted differences in malaria-related clinical outcomes in Kenyan children with high-titer IgG antibodies to AMA-1, EBA-175, or MSP-119 (AU>2) as compared to children without these antibodies.
| Antigens | No. with Ab/total no. | Clinical outcome | Analysis* | Relative risk in children with high-titer antibodies (95% CI) | P |
|---|---|---|---|---|---|
| AMA-1 | 54/87 | Clinical malaria | Hazard ratio | 0.77 (0.40, 1.48) | 0.433 |
| Cumulative malaria incidence | Incidence rate ratio | 0.88 0(.50, 1.57) | 0.683 | ||
|
| |||||
| EBA-175 | 44/87 | Clinical malaria | Hazard ratio | 0.48 (0.24, 0.95) | 0.034 |
| Cumulative malaria incidence | Incidence rate ratio | 0.50 (0.31,0.81) | 0.005 | ||
|
| |||||
| MSP-119 | 18/72 | Clinical malaria | Hazard ratio | 0.46 (0.13, 1.57) | 0.215 |
| Cumulative malaria incidence | Incidence rate ratio | 0.50 (0.18, 1.31) | 0.158 | ||
|
| |||||
| AMA-1, EBA-175 & MSP-119 | 9/72 | Clinical malaria | Hazard ratio | 0.25 (0.03, 1.94) | 0.185 |
| Cumulative malaria incidence | Incidence rate ratio | 0.16 (0.02, 1.20) | 0.07 | ||
Values adjusted for village, age, bed net use and parasitemia at time of enrolment. Risk of clinical malaria assessed by Cox regression and cumulative malaria incidence by negative binomial regression.
Ab = antibody. 95% CI = 95% confidence interval.
Cox regression also showed a trend toward reduction in risk of clinical malaria with log increase in antibodies to EBA-175 (HR 0.81, 95% CI 0.65, 1.01, P=0.07) and MSP-119 (HR 0.73. 95% CI 0.53, 1.00, P=0.06) but not AMA-1 (HR 1.05, 95% CI 0.79, 1.41, P=0.71). The presence of high-titer antibodies to all three antigens was associated with the lowest risk of clinical malaria (HR 0.26, 95% CI 0.03, 1.94, P=0.18) and the greatest decrease in malaria incidence (IRR 0.16, 95 % CI 0.02, 1.20, P=0.07), but only nine children had high-titer antibodies to all three antigens, and these decreases in risk did not achieve statistical significance. Protection associated with all three antigens was due to the presence of high-titer antibodies to MSP-119 and EBA-175, as children with antibodies to these antigens had the same decrease in risk (HR 0.29, 95% CI 0.06, 1.40, P=0.12) as those with antibodies to all three antigens. Only 15 children reached the secondary endpoint of high-density parasitemia with clinical malaria, so although all hazard ratios were <1, no significant associations with protection were seen for antibodies to any antigen (AMA-1, HR 0.87, 95% CI, 0.26, 2.87, P=0.82; EBA-175, HR 0.42, 95% CI 0.12, 1.50, P=0.18; MSP-119, HR 0.88, 0.18, 4.20, P=0.87).
Discussion
In the present study, we document that high-titer antibodies to EBA-175 are associated with protection from clinical malaria in children in a malaria holoendemic area of Kenya. Importantly, we found that even in this relatively small area (<12 km2), there were important local differences in frequency of clinical malaria and therefore exposure to blood-stage P. falciparum, and that controlling for these differences in exposure resulted in differing estimates of associations with protection from clinical malaria. Prior to adjustment for exposure, significant associations were seen with protection for high-titer antibodies to MSP-119 but not EBA-175, whereas after adjustment for exposure, significant associations were seen for high-titer antibodies to EBA-175 but not MSP-119. The changes seen in the associations with protection to high-titer antibodies to EBA-175 with adjustment for exposure demonstrate the importance of assessing and controlling for exposure in studies of associations with protection against clinical malaria, even in areas presumed to have relatively homogenous exposure. Finally, there was evidence suggestive of additive protective association in children with high-titer antibodies to both EBA-175 and MSP-119, though the study did not have power to detect significant differences in protection for the small number of children (n=13) with high-titer antibodies to both antigens.
The present study provides further evidence that high-titer antibodies to EBA-175 are associated with protection from clinical malaria, though the precise region (II or III-V) that is most associated with protection may vary by area of study. A recent study from Papua New Guinea demonstrated that antibodies to EBA-175 region II and regions III-V were strongly associated with protection from clinical malaria and high-density parasitemia [15]. In the Papua New Guinea study, as in the present study, high-titer antibodies to EBA-175 were assessed (highest tertile compared to lowest tertile in the Papua New Guinea study, AU>2 in the present study). In both studies, high-titer antibodies, as opposed to presence of any amount of antibody, were most strongly associated with protection from clinical malaria. Earlier studies from the Gambia [21] and Kenya [16, 22] did not show an association between the presence of antibodies to EBA-175 region II and protection from clinical malaria, but in the study from the Gambia, high-titer antibodies (OD>1.5) to EBA-175 region II did show an association with protection from clinical malaria [21]. These findings together suggest that high concentrations of antibodies to EBA-175, as opposed to just presence of antibodies to EBA-175, are required for protection against clinical malaria.
There are number of reasons to believe that antibodies to EBA-175 could be involved in protection from clinical malaria in children in malaria endemic areas. EBA-175 binding to human erythrocytes occurs on sialic acid residues of O-linked tetrasaccharides on glycophorin A and is involved in junction formation. This marks the initial step of erythrocyte invasion, and blocking this event may prevent invasion [23, 24] of both sialic acid dependent and independent strains[25]. Invasion inhibition of human erythrocytes has been shown in vitro to be blocked with antibodies to EBA-175 raised in murine models[26], and studies in Aotus monkeys have demonstrated that antibodies to EBA-175 suppress parasitemia both in vivo and in vitro [27]. Early studies suggested that EBA-175 might have a role in sporozoite invasion of hepatocytes as well as merozoite invasion of erythrocytes[28, 29], since it is also expressed in the pre-erythrocytic stage of the malarial life cycle. However, more recent work has shown that EBA-175 is not expressed in parasites infecting hepatocytes until 6 days after inoculation and therefore does not likely play a role in sporozoite invasion [30]. Antibodies to EBA-175 region II have been most carefully studied because this region mediates erythrocyte binding [31]. However, the study in Papua New Guinea showed stronger association with protection from clinical malaria with antibodies to regions III-V, so further assessment of the functional characteristics these regions and of protective associations of antibodies to these regions in malaria endemic areas is indicated.
Antibodies to MSP-119 have been associated with protection from clinical malaria in some studies [13, 14, 32] but not others [10–12, 33]. These studies include a study done by our group in highland Kenya which showed that invasion-inhibitory antibodies to MSP-119, but not total IgG antibodies to MSP-119, were associated with protection from clinical malaria [34]. Similarly, some studies have documented associations between antibodies or antibody concentrations to AMA-1 and protection from clinical malaria[6, 8, 9, 16], while other have not [7, 13, 35]. Potential reasons for these conflicting results include differences in the intensity and stability of transmission, IgG subclass switching, allelic variation of specific antigens, and differences in study design[4, 36]. Antibodies to MSP-119 may have more fine specificity for antigen variants than antibodies to other antigens[37, 38], and this may explain some of the differences in findings for this antigen. Similarly, differences in strain (3D7 vs. FVO) for AMA-1 made a difference in associations with protection for this antigen in one study [39]. We were not able to genotype parasites at the time of clinical malaria in the present study to assess for the importance of allele-specific protection, but plan to do so in future studies.
The IgG subclass profile in the present study described a relative absence of both IgG2 and IgG4 antibodies consistent with similar studies of the IgG subclasses[21, 40, 41]. The distinct role of each IgG subclass antibody is not well understood. However there is evidence that the cytophilic subclasses, IgG1 and IgG3, are correlated with protection from clinical disease and are thought work through activation of monocytes through the Fc receptors [42–44]. IgG2 and IgG4 have been proposed to block opsonization and phagocytosis by competitively blocking these targets[45] and this may explain the decreased frequency of these antibodies in this study. Interestingly, some studies have found IgG1 may be more important for protection in younger children of endemic areas and malaria naïve adults while IgG3 is more important in older children[46]. This suggests an age and exposure relationship with the IgG subclass antibodies in the development of resistance to disease.
The present study also highlights the importance of assessing exposure when conducting studies of antibody association with protection from clinical malaria. Surrogate markers of exposure in earlier studies have included antibodies to schizont extract [16] and proximity to swamp areas[47]. In the present study, we used the practical marker of village, as there were no surrounding swamps or other obvious risk factors. The marker was validated by the strong correlation of village with risk of clinical malaria, and adjustment for this marker of exposure significantly altered estimates of associations with protection from clinical malaria. Adjustment for exposure allows a study to control for differences in subsequent risk (which will be higher in areas of greater exposure) and provide some level of adjustment for non-specific immunity, providing greater confidence that the associations seen are in fact specific to the antibodies assessed. The present study demonstrates the importance of adjustment for exposure even in areas without obvious geographic variation.
In summary, the study findings provide further evidence that high-titer antibodies (AU>2) to EBA-175 may be a useful correlate of protective immunity in children in malaria endemic areas, and demonstrate the importance of adjustment for exposure in studies of antibody association with protection against clinical malaria. The study findings also support a possible additive effect of antibodies to EBA-175 and MSP-119 in association with protection from clinical malaria. These findings support the growing body of evidence for EBA-175 as a malaria vaccine candidate antigen, and further support development of multiple antigen vaccines.
Acknowledgments
We acknowledge the work of Jackson Abuya in field supervision and microscopy, and thank the study field assistants and the study participants for their involvement in the study. We thank the Malaria Research and Reference Reagent Resource Center (MR-4, Manassas, VA) for providing the recombinant MSP-119, donated by David Kaslow. This study was supported in part by grants U01 AI056270 to Chandy C. John and R01AI043906 to James W. Kazura and a NIAD-NIH contract N01-AI05421. This study was published with the permission of the Director, Kenya Medical Research Institute. This research was supported in part by the Intramural Research Program of the NIH, Malaria Vaccine Development Branch, National Institute for Allergy and Infectious Diseases.
References
- 1.Aregawi M, Cibulskis R, Kita Y, Otten M, Williams R. World Malaria Report 2010. Geneva: World Health Organization; 2010. pp. 90–92. [Google Scholar]
- 2.Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–7. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 3.McGregor IA. The Passive Transfer of Human Malarial Immunity. The American journal of tropical medicine and hygiene. 1964;13(SUPPL):237–9. doi: 10.4269/ajtmh.1964.13.237. [DOI] [PubMed] [Google Scholar]
- 4.Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite immunology. 2006;28:51–60. doi: 10.1111/j.1365-3024.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 5.Good MF. Towards a blood-stage vaccine for malaria: are we following all the leads? Nature reviews. 2001;1:117–25. doi: 10.1038/35100540. [DOI] [PubMed] [Google Scholar]
- 6.Stanisic DI, Richards JS, McCallum FJ, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infection and immunity. 2009;77:1165–74. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nebie I, Diarra A, Ouedraogo A, et al. Humoral responses to Plasmodium falciparum blood-stage antigens and association with incidence of clinical malaria in children living in an area of seasonal malaria transmission in Burkina Faso, West Africa. Infection and immunity. 2008;76:759–66. doi: 10.1128/IAI.01147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polley SD, Mwangi T, Kocken CH, et al. Human antibodies to recombinant protein constructs of Plasmodium falciparum Apical Membrane Antigen 1 (AMA1) and their associations with protection from malaria. Vaccine. 2004;23:718–28. doi: 10.1016/j.vaccine.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Fowkes FJ, Richards JS, Simpson JA, Beeson JG. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS Med. 2010;7:e1000218. doi: 10.1371/journal.pmed.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murhandarwati EE, Wang L, Black CG, et al. Inhibitory antibodies specific for the 19-kilodalton fragment of merozoite surface protein 1 do not correlate with delayed appearance of infection with Plasmodium falciparum in semi-immune individuals in Vietnam. Infection and immunity. 2009;77:4510–7. doi: 10.1128/IAI.00360-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitua AY, Urassa H, Wechsler M, et al. Antibodies against Plasmodium falciparum vaccine candidates in infants in an area of intense and perennial transmission: relationships with clinical malaria and with entomological inoculation rates. Parasite immunology. 1999;21:307–17. doi: 10.1046/j.1365-3024.1999.00230.x. [DOI] [PubMed] [Google Scholar]
- 12.Nwuba RI, Sodeinde O, Anumudu CI, et al. The human immune response to Plasmodium falciparum includes both antibodies that inhibit merozoite surface protein 1 secondary processing and blocking antibodies. Infection and immunity. 2002;70:5328–31. doi: 10.1128/IAI.70.9.5328-5331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodoo D, Aikins A, Kusi KA, et al. Cohort study of the association of antibody levels to AMA1, MSP119, MSP3 and GLURP with protection from clinical malaria in Ghanaian children. Malar J. 2008;7:142. doi: 10.1186/1475-2875-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branch OH, Udhayakumar V, Hightower AW, et al. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kiloDalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. The American journal of tropical medicine and hygiene. 1998;58:211–9. doi: 10.4269/ajtmh.1998.58.211. [DOI] [PubMed] [Google Scholar]
- 15.Richards JS, Stanisic DI, Fowkes FJ, et al. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis. 2010;51:e50–60. doi: 10.1086/656413. [DOI] [PubMed] [Google Scholar]
- 16.Osier FH, Fegan G, Polley SD, et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infection and immunity. 2008;76:2240–8. doi: 10.1128/IAI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John CC, Moormann AM, Sumba PO, et al. Gamma interferon responses to Plasmodium falciparum liver-stage antigen 1 and thrombospondin-related adhesive protein and their relationship to age, transmission intensity, and protection against malaria. Infection and immunity. 2004;72:5135–42. doi: 10.1128/IAI.72.9.5135-5142.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.John CC, Tande AJ, Moormann AM, et al. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. The Journal of infectious diseases. 2008;197:519–26. doi: 10.1086/526787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaslow DCHG, Kumar S. Expression and antigenicity of Plasmodium falciparum major merozoite surface protein (MSP1(19)) variants secreted from Saccharomyces cerevisiae. Mol Biochem Parasitol. 1994:283–289. doi: 10.1016/0166-6851(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 20.John CC, Moormann AM, Pregibon DC, et al. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. The American journal of tropical medicine and hygiene. 2005;73:222–8. [PubMed] [Google Scholar]
- 21.Okenu DM, Riley EM, Bickle QD, et al. Analysis of human antibodies to erythrocyte binding antigen 175 of Plasmodium falciparum. Infection and immunity. 2000;68:5559–66. doi: 10.1128/iai.68.10.5559-5566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohas EA, Adams JH, Waitumbi JN, et al. Measurement of antibody levels against region II of the erythrocyte-binding antigen 175 of Plasmodium falciparum in an area of malaria holoendemicity in western Kenya. Infection and immunity. 2004;72:735–41. doi: 10.1128/IAI.72.2.735-741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vera-Bravo R, Valbuena JJ, Ocampo M, et al. Amino terminal peptides from the Plasmodium falciparum EBA-181/JESEBL protein bind specifically to erythrocytes and inhibit in vitro merozoite invasion. Biochimie. 2005;87:425–36. doi: 10.1016/j.biochi.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 24.O’Donnell RA, Hackett F, Howell SA, et al. Intramembrane proteolysis mediates shedding of a key adhesin during erythrocyte invasion by the malaria parasite. J Cell Biol. 2006;174:1023–33. doi: 10.1083/jcb.200604136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narum DL, Haynes JD, Fuhrmann S, et al. Antibodies against the Plasmodium falciparum receptor binding domain of EBA-175 block invasion pathways that do not involve sialic acids. Infection and immunity. 2000;68:1964–6. doi: 10.1128/iai.68.4.1964-1966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sim BK, Orlandi PA, Haynes JD, et al. Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J Cell Biol. 1990;111:1877–84. doi: 10.1083/jcb.111.5.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones TR, Narum DL, Gozalo AS, et al. Protection of Aotus monkeys by Plasmodium falciparum EBA-175 region II DNA prime-protein boost immunization regimen. The Journal of infectious diseases. 2001;183:303–312. doi: 10.1086/317933. [DOI] [PubMed] [Google Scholar]
- 28.Florens L, Washburn MP, Raine JD, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419:520–6. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 29.Silvie O, Franetich JF, Charrin S, et al. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. The Journal of biological chemistry. 2004;279:9490–6. doi: 10.1074/jbc.M311331200. [DOI] [PubMed] [Google Scholar]
- 30.Sim BK, Narum DL, Luu T, et al. Delineation of Stage Specific Expression of Plasmodium falciparum EBA-175 by Biologically Functional Region II Monoclonal Antibodies. doi: 10.1371/journal.pone.0018393. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sim BK, Chitnis CE, Wasniowska K, Hadley TJ, Miller LH. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994;264:1941–4. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 32.al-Yaman F, Genton B, Kramer KJ, et al. Assessment of the role of naturally acquired antibody levels to Plasmodium falciparum merozoite surface protein-1 in protecting Papua New Guinean children from malaria morbidity. The American journal of tropical medicine and hygiene. 1996;54:443–8. doi: 10.4269/ajtmh.1996.54.443. [DOI] [PubMed] [Google Scholar]
- 33.Dodoo D, Theander TG, Kurtzhals JA, et al. Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infection and immunity. 1999;67:2131–7. doi: 10.1128/iai.67.5.2131-2137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.John C, O’Donnell R, Sumba P, et al. Evidence That Invasion-Inhibitory Antibodies Specific for the 19-kDa Fragment of Merozoite Surface Protein-1 (MSP-119) Can Play a Protective Role against Blood-Stage Plasmodium falciparum Infection in Individuals in a Malaria Endemic Area of Africa. The Journal of Immunology. 2004 doi: 10.4049/jimmunol.173.1.666. [DOI] [PubMed] [Google Scholar]
- 35.Gray JC, Corran PH, Mangia E, et al. Profiling the antibody immune response against blood stage malaria vaccine candidates. Clin Chem. 2007;53:1244–53. doi: 10.1373/clinchem.2006.081695. [DOI] [PubMed] [Google Scholar]
- 36.Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med. 1999;5:340–3. doi: 10.1038/6560. [DOI] [PubMed] [Google Scholar]
- 37.Corran PH, O’Donnell RA, Todd J, et al. The fine specificity, but not the invasion inhibitory activity, of 19-kilodalton merozoite surface protein 1-specific antibodies is associated with resistance to malarial parasitemia in a cross-sectional survey in The Gambia. Infection and immunity. 2004;72:6185–9. doi: 10.1128/IAI.72.10.6185-6189.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okech BA, Corran PH, Todd J, et al. Fine specificity of serum antibodies to Plasmodium falciparum merozoite surface protein, PfMSP-1(19), predicts protection from malaria infection and high-density parasitemia. Infection and immunity. 2004;72:1557–67. doi: 10.1128/IAI.72.3.1557-1567.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osier FH, Weedall GD, Verra F, et al. Allelic diversity and naturally acquired allele-specific antibody responses to Plasmodium falciparum apical membrane antigen 1 in Kenya. Infection and immunity. 2010;78:4625–33. doi: 10.1128/IAI.00576-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehrizi AA, Zakeri S, Salmanian AH, Sanati MH, Djadid ND. IgG subclasses pattern and high-avidity antibody to the C-terminal region of merozoite surface protein 1 of Plasmodium vivax in an unstable hypoendemic region in Iran. Acta Trop. 2009;112:1–7. doi: 10.1016/j.actatropica.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Wickramarachchi T, Illeperuma RJ, Perera L, et al. Comparison of naturally acquired antibody responses against the C-terminal processing products of Plasmodium vivax Merozoite Surface Protein-1 under low transmission and unstable malaria conditions in Sri Lanka. Int J Parasitol. 2007;37:199–208. doi: 10.1016/j.ijpara.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Taylor RR, Allen SJ, Greenwood BM, Riley EM. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. The American journal of tropical medicine and hygiene. 1998;58:406–13. doi: 10.4269/ajtmh.1998.58.406. [DOI] [PubMed] [Google Scholar]
- 43.Aribot G, Rogier C, Sarthou JL, et al. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, west Africa) The American journal of tropical medicine and hygiene. 1996;54:449–57. doi: 10.4269/ajtmh.1996.54.449. [DOI] [PubMed] [Google Scholar]
- 44.Ferreira MU, Kimura EA, Katzin AM, et al. The IgG-subclass distribution of naturally acquired antibodies to Plasmodium falciparum, in relation to malaria exposure and severity. Ann Trop Med Parasitol. 1998;92:245–56. doi: 10.1080/00034989859807. [DOI] [PubMed] [Google Scholar]
- 45.Bouharoun-Tayoun H, Druilhe P. Antibodies in falciparum malaria: what matters most, quantity or quality? Mem Inst Oswaldo Cruz. 1992;87 (Suppl 3):229–34. doi: 10.1590/s0074-02761992000700038. [DOI] [PubMed] [Google Scholar]
- 46.Ndungu FM, Bull PC, Ross A, et al. Naturally acquired immunoglobulin (Ig)G subclass antibodies to crude asexual Plasmodium falciparum lysates: evidence for association with protection for IgG1 and disease for IgG2. Parasite immunology. 2002;24:77–82. doi: 10.1046/j.0141-9838.2001.00440.x. [DOI] [PubMed] [Google Scholar]
- 47.Clark TD, Greenhouse B, Njama-Meya D, et al. Factors determining the heterogeneity of malaria incidence in children in Kampala, Uganda. The Journal of infectious diseases. 2008;198:393–400. doi: 10.1086/589778. [DOI] [PubMed] [Google Scholar]