Figure 9.
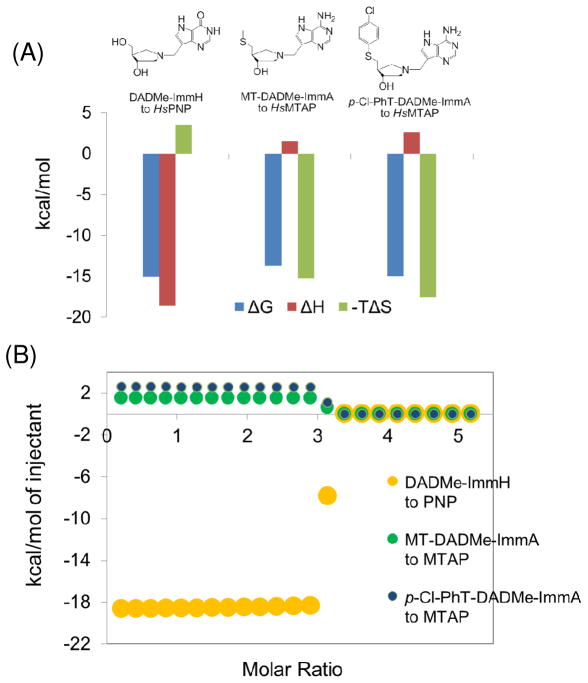
Binding thermodynamics of PNP and MTAP. The thermodynamic signatures of PNP (12) and MTAP are compared for the binding of DADMe-ImmH (Ki of 8.5 pM) and MT-DADMe-ImmA (Ki of 90 pM) in panel A. The binding energy of PNP comes completely from favorable enthalpy with an entropic penalty. The tight binding of MTAP is driven by entropy with a minor enthalpy penalty. Thermodynamic titration data for PNP and MTAP are compared in the simulation in panel B.