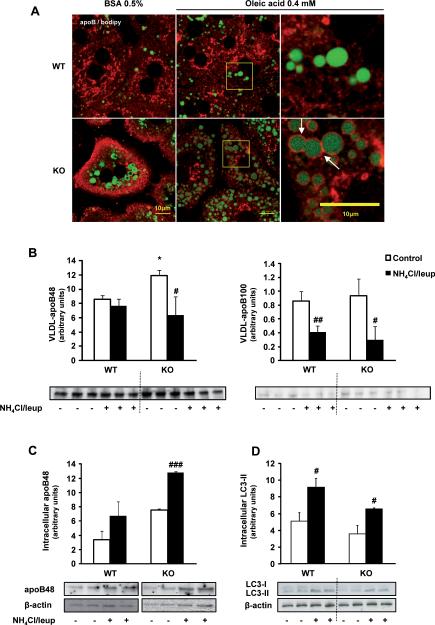
Fig. 5. Increased VLDL-apoB secretion in 3-month-old MAT1A-KO mice is linked to altered localization of apoB in hepatocytes but not to autophagy.
(A) For confocal laser immunofluorescence analysis of apoB localization in hepatocytes, primary 3-month-old wild type (WT) and MAT1A-knockout (MAT1A-KO) mice hepatocytes were incubated 4 hours in coverslips in DMEM complemented with 0.5% fatty acid free BSA or 0.4 mM oleic acid in 0.5% BSA, and afterwards PBS washed and fixed with 3.7% formaldehyde. After permeabilization (5% Triton X-100), cells were blocked (10% foetal bovine serum in PBS) and double labeled with anti-apoB antibody (red) and BODIPY® 493/503 (green). (B–D) Hepatocytes were incubated 24 hours in DMEM without (control, □) or with (■) a mixture of the lysosomal inhibitors leupeptin (100 μM) and ammonium chloride (20 μM). (B) VLDL were isolated from the medium as detailed in material and methods and the VLDL-apoB quantity was analyzed by immunoblotting. (C) Intracellular apoB48 content was assessed by immunoblotting using β-actin as normalizer (apoB100 was not detected). (D) Autophagy was assessed by analysis of the expression of the microtubule-associated proteins, light chain (LC)3-I and LC3-II by immunoblotting. Statistical differences versus WT mice are denoted by *p<0.05 and versus control hepatocytes are denoted by #p<0.05, ##p<0.01 and ###p<0.001 (Student's t test).