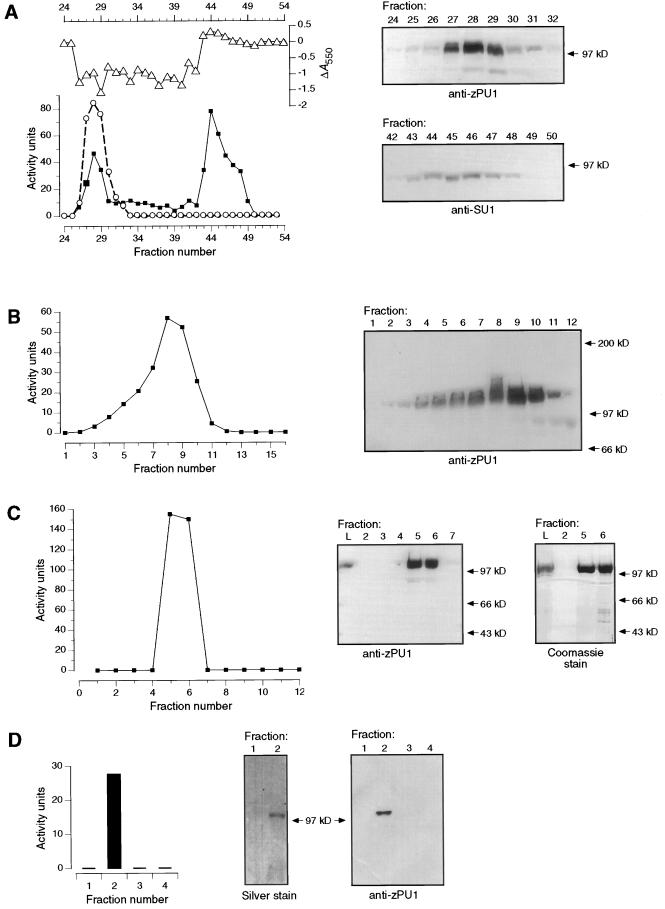
Figure 5.
A, Q-Sepharose chromatography. Fractions eluted from the column were assayed for DBE activity using pullulan (○) or amylopectin (▪) as a substrate. Products of the amylopectin reaction were complexed with iodine, and the change in A550 value relative to untreated substrate was plotted (▵). Activity units for the amylopectin digestion are microgram maltose equivalents produced after a 2-h incubation of substrate with 100 μL of protein fraction. Activity units for the pullulan digestion are microgram maltotriose equivalents produced after a 2-h incubation of the substrate with 50 μL of the protein fraction. Fractions with DBE activity were subjected to immunoblot analysis with anti-ZPU1 or anti-SU1 antiserum, as indicated (right-hand panels). B, Gel-filtration chromatography. The peak fractions of pullulanase-type activity from Q-Sepharose columns were pooled, concentrated, and applied to a Sephacryl S-200 superfine gel-permeation column. DBE activity in the fractions eluted from this column was assayed using pullulan as the substrate; activity units are as described for A. Fractions were also assayed for the presence of ZPU1 by immunoblot analysis (right-hand panel). C, Mono-Q chromatography. The peak fractions (7–11) of pullulanase-type activity from the Sephacryl S-200 column were pooled, concentrated, and applied to a Pharmacia fast-protein liquid chromatography Mono-Q column. DBE activity in fractions eluted from this column was assayed using pullulan as the substrate; activity units are as described for A. Fractions were assayed for the presence of ZPU1 in immunoblots (right-hand panels). D, Affinity chromatography. The peak fractions of pullulanase-type activity from Q-Sepharose columns were pooled, concentrated, and applied to a column containing epoxy-activated Sepharose conjugated with cyclohexa-amylose. DBE activity in the four fractions eluted from this column was assayed using pullulan as the substrate; activity units are as described for A. Proteins from two of the fractions were separated by SDS-PAGE and the gel was silver stained; a duplicate gel was subjected to immunoblot analysis with anti-ZPU1 (right-hand panels).