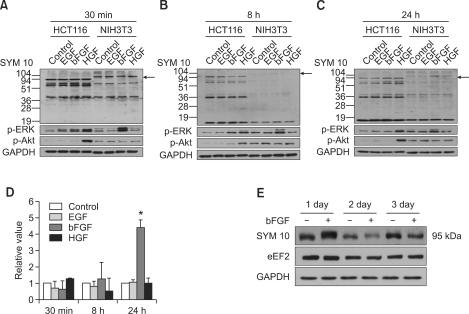
Figure 1.
Symmetric dimethylation of arginine on a 95-kDa protein is induced by bFGF in NIH3T3 cells. Protein arginine methylation profiles of NIH3T3 and HCT116 cells produced at (A) 30 min, (B) 8 h, and (C) 24 h after the administration of EGF, bFGF, or HGF with concurrent changes in the levels of p-ERK and p-Akt activation. Symmetric dimethylation of arginine on a 95-kDa protein is induced by bFGF 24 h after administration into NIH3T3 cells. ERK1/2 was activated 30 min after bFGF administration, which decreased gradually thereafter, while the level of Akt activation remained unchanged during the period. Cells were cultured in the absence or presence of EGF, bFGF, or HGF, and cell lysates were prepared at 30 min, 8 h, and 24 h. Western blot analysis was performed with anti-symmetric dimethylarginine antibody SYM10 and anti-p-ERK1/2 or anti-p-Akt antibodies. GAPDH was used as a loading control. Protein bands were visualized using enhanced chemiluminescence. (D) Bar graph showing the change in levels of symmetric arginine dimethylation of a 95-kDa protein after the administration of growth factors. Mean and standard deviations were obtained from three independent experiments. Statistically significant differences as determined by the Wilcoxon test were set at *P < 0.001. (E) The change in the levels of symmetric arginine dimethylation of a 95-kDa protein and eEF2 expression in NIH3T3 cells during the three-day period after the administration of bFGF. Cell lysates were obtained at the indicated time points of 1, 2 and 3 days, and Western blots were conducted using SYM10 and anti-eEF2 antibody as in (A). Arrows indicate the symmetrically arginine-dimethylated 95-kDa protein.