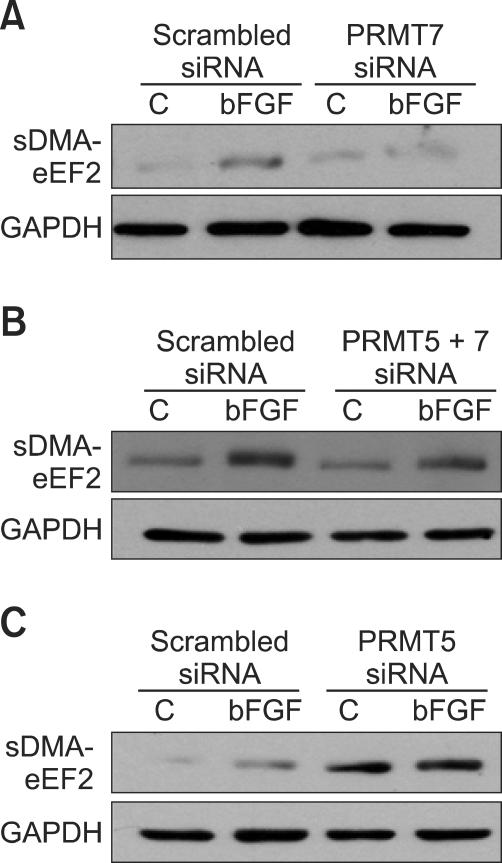
Figure 4.
PRMT7 is responsible for symmetric dimethylation of arginine on eEF2 induced by bFGF, while PRMT5 appears to play a coordinating role. (A) The inhibition of PRMT7 by siRNA resulted in the complete loss of the bFGF-induced symmetric arginine dimethylation of eEF2. The levels of symmetric dimethylation of arginine on eEF2 and α-SMA expressions were not correlated to each other. NIH3T3 cells were transfected with PRMT7 siRNA for 4 days and treated with bFGF for 24 h. Cell lysates were prepared, and the RNA level of PRMT7 was analyzed using real-time quantitative PCR. Western blot analysis was conducted with SYM10 and anti-α-SMA antibodies. Anti-α-SMA Western blot was used to determine the interrelationship between the levels of symmetric dimethylation of arginine on eEF2 and the expression of the fibroblast differentiation marker, α-SMA. Anti-GAPDH antibody was used as a loading control. (B) PRMT5 inhibition remarkably increases the amount of sDMA on eEF2 and negates the regulatory effect of bFGF on the symmetric arginine dimethylation of eEF2. NIH3T3 cells were transfected with PRMT5 siRNA for 4 days and treated with bFGF for 24 h. Cell lysates were prepared, and Western blot analysis was conducted as in (A). (C) The simultaneous inhibition of PRMT7 and PRMT5 suppressed the bFGF-induced symmetric arginine dimethylation of eEF2. NIH3T3 cells were co-transfected with PRMT7 and PRMT5 siRNAs for 4 days and treated with bFGF for 24 h. Cell lysates were prepared, and Western blot analysis was conducted as in (A).