Table 2.
Substrate Scope of Room Temperature Pd/Ru-Catalyzed C–H Arylation of 2-Arylpyridine Derivatives
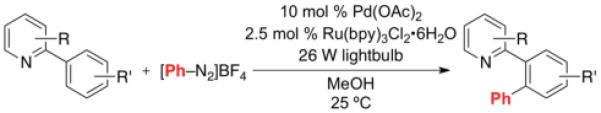
| Entry | Substrate | Product | Isolated Yielda |
|---|---|---|---|
| 1 b |
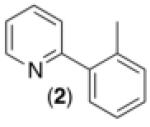
|
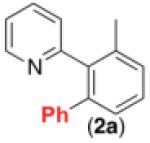
|
76% |
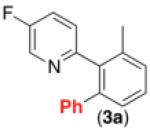
| 2c,d |
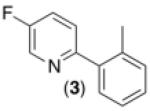
|
|
66% |
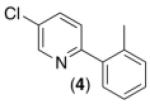
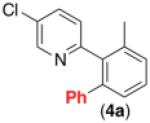
| 3b,d,e,f |
|
|
62% |
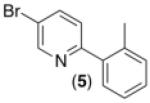
| 4b,c,d,e,f |
|
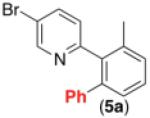
|
63% |
| 5d,g |
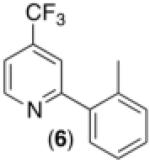
|
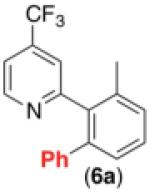
|
59% |
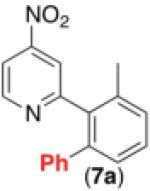
| 6c,d,e,h |
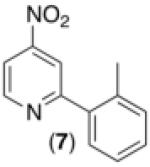
|
|
47% |
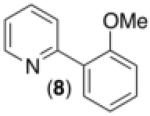
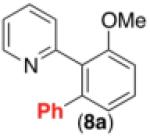
| 7d,f |
|
|
69% |
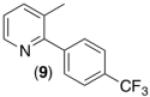
| 8c,d,e,g |
|
|
60% |
General procedure: Substrate (1.0 equiv), Pd(OAc)2 (0.1 equiv), Ru(bpy)3Cl2•6H2O (0.025 equiv), PhN2BF4 (4.0 equiv), MeOH (0.1 M in substrate), rt, 4 h, 26 W compact fluorescent light bulb.
Ag2CO3 (0.1 equiv) used as an additive.
General conditions but with 0.15 equiv of Pd(OAc)2.
MeOH (0.05 M in substrate).
5.0 equiv of PhN2BF4
Reaction run for 10 h.
Reaction run for 8 h.
Reaction run for 9.5 h.
