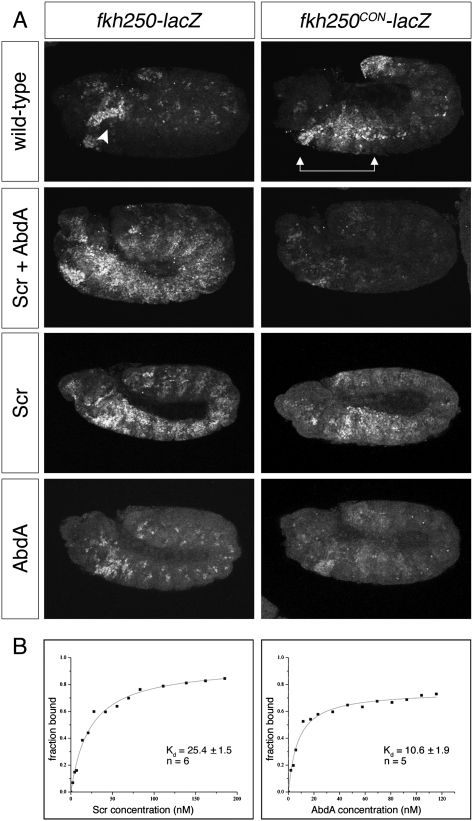
Figure 1.
AbdA dominance over Scr relies on an Exd-dependent DNA-binding mechanism. (A) Embryos carrying fkh250-lacZ (left panels) or fkh250CON-lacZ (right panels) stained for β-galactosidase (β-gal). In wild-type embryos, endogenous Scr activates fkh250-lacZ in PS2 (arrowhead), while fkh250CON-lacZ is activated by Scr, Antp, and Ubx from PS2 to PS6 (arrows) and is repressed by AbdA in the abdominal segments. Ectopic expression of Scr throughout the embryo, alone or in combination with AbdA, results in widespread activation of fkh250-lacZ. In contrast, fkh250CON-lacZ is repressed when Scr and AbdA are both ectopically expressed. (B) Representative in vitro saturation binding experiments and dissociation constant (Kd in nanomolar) fits are shown for Scr–Exd (left) and AbdA–Exd (right) binding to fkh250CON. AbdA–Exd dimers bound more tightly to fkh250CON than Scr–Exd did, supporting a model in which AbdA dominance depends on cofactor-dependent DNA binding. All assays were performed in the presence of Exd–HthHM. The reported Kds represent the averages and standard error of the means of repeated measurements (n = 5 for AbdA, and n = 6 for Scr) (see the Materials and Methods).