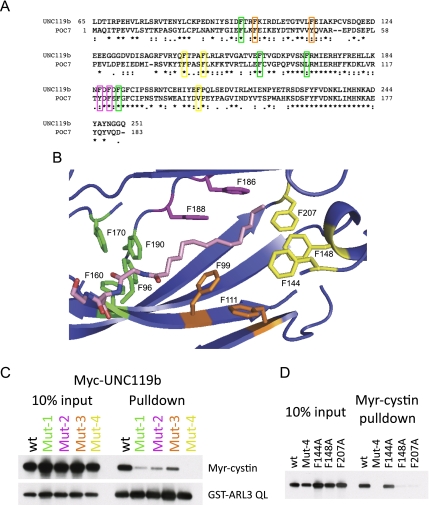
Figure 3.
Mutations in the highly conserved phenyl groove of UNC119 disrupt myristoyl–peptide binding. (A) Sequence alignment of human UNC119b and the Chlamydomonas homolog POC7. Phenylalanine residues mutated in C are indicated and color-coded. (B) Structural model of UNC119a and the myristoylated N-terminal three amino acids of cystin based on the UNC119a crystal structure 3GQQ. The four groups of conserved phenylalanines mutagenized in C are shown and color-coded. Residue numbers correspond to the UNC119b sequence. Portions of the structure were removed to allow visualization of the myristoyl-binding pocket. (C, top immunoblot) Biotinylated Myr-cystin-binding assays were performed as in Figure 2E only using in vitro translated wild-type (wt) or mutant Myc-tagged UNC119b. The bottom immunoblot shows the results of a binding reaction between GST-ARL3 QL and in vitro translated Myc-tagged UNC119b. (D) Biotinylated Myr-cystin-binding assays were performed as in Figure 2E only using in vitro translated wild-type (wt) or mutant Myc-tagged UNC119b.