Abstract
Two epidemic modeling studies of inhalational tularemia were identified in the published literature, both demonstrating the high number of potential casualties that could result from a deliberate aerosolized release of the causative agent in an urban setting. However, neither study analyzed the natural history of inhalational tularemia nor modeled the relative merits of different mitigation strategies. We first analyzed publicly available human/primate experimental data and reports of naturally acquired inhalational tularemia cases to better understand the epidemiology of the disease. We then simulated an aerosolized release of the causative agent, using airborne dispersion modeling to demonstrate the potential number of casualties and the extent of their spatial distribution. Finally, we developed a public health intervention model that compares 2 mitigation strategies: targeting antibiotics at symptomatic individuals with or without mass distribution of antibiotics to potentially infected individuals. An antibiotic stockpile that is sufficient to capture all areas where symptomatic individuals were infected is likely to save more lives than treating symptomatic individuals alone, providing antibiotics can be distributed rapidly and their uptake is high. However, with smaller stockpiles, a strategy of treating symptomatic individuals alone is likely to save many more lives than additional mass distribution of antibiotics to potentially infected individuals. The spatial distribution of symptomatic individuals is unlikely to coincide exactly with the path of the dispersion cloud if such individuals are infected near their work locations but then seek treatment close to their homes. The optimal mitigation strategy will depend critically on the size of the release relative to the stockpile level and the effectiveness of treatment relative to the speed at which antibiotics can be distributed.
The authors analyzed publicly available data and reports of inhalational tularemia cases to better understand the epidemiology of the disease. They then simulated an aerosolized release of the causative agent, using airborne dispersion modeling to demonstrate the potential number of casualties and the extent of their distribution. Finally, they developed a public health intervention model that compares 2 mitigation strategies: targeting antibiotics at symptomatic individuals with or without mass distribution of antibiotics to potentially infected people.
Tularemia is a zoonosis caused by Francisella tularensis, a bacterium found naturally in diverse animal hosts and contaminated environments throughout much of North America and Eurasia.1 Vectors such as ticks, flies, and mosquitoes are known to transmit the infection from animal reservoirs to humans.2 Other common modes of human infection include handling infectious animal tissues and inhaling infective aerosols.1 In 1978 a cluster of inhalational tularemia cases occurred in Martha's Vineyard, Massachusetts, after a wet dog was believed to have aerosolized F. tularensis while shaking itself inside the cottage where all 7 cases were staying.3,4 Other notable inhalational tularemia outbreaks include more than 600 cases in Sweden in 1966,5 where contaminated hay was being farmed, and again in Martha's Vineyard in 1990, where 2 adolescent males accidentally ran over a dead rabbit with a lawnmower.6
Aerosolized F. tularensis is also considered to have potential as a biological weapon. It was studied at Japanese germ warfare research units prior to and during World War II and was examined for military purposes by the U.S. and the Soviet Union during the Cold War.1 A vaccine was developed that partially protected against respiratory challenges, but it is currently unlicensed and has not been recommended in a mass casualty situation.1 Various antibiotic regimens are effective as postexposure prophylaxis and treatment for tularemia and would likely comprise the first line of public health intervention in response to a deliberate release of F. tularensis.1 Although secondary transmission has been reported,7 human-to-human spread of tularemia is generally considered extremely rare.
In October 2003, F. tularensis was detected by an environmental monitoring system, BioWatch, in Houston, Texas. Although the alert was caused by naturally occurring background organism levels, the need for ongoing bioterrorism consequence management planning was highlighted.8 Important aspects of public health preparedness include quantitative assessments of disease kinetics and the evaluation of mitigation strategies via mathematical modeling.
Two previous modeling studies of tularemia have estimated that a deliberate release of F. tularensis into unprotected populations of 100,000 and 5 million people would result in approximately 82,500 and 250,000 symptomatic individuals, respectively.9,10 However, neither study analyzed the natural history of inhalational tularemia nor evaluated the importance of considering how potential mitigation strategies might affect the public health outcomes. A spatial back-calculation method that can estimate the location and spatial extent of a covert pathogen release using information on early cases and meteorological data has recently been developed.11,12 However, with limited data available to validate such a statistical tool, it is an open challenge to gauge how the methodology would perform in an actual emergency situation.
Therefore, it is also important to consider alternative strategies for mitigating a biological release of F. tularensis. Here, we begin by evaluating key epidemiologic determinants of inhalational tularemia based on known cases and also human/primate experimental data. We then model a release of F. tularensis in an urban environment and the subsequent disease progression in the civilian population. Finally, we compare the effectiveness of potential public health responses involving the targeting of antibiotics at symptomatic individuals with or without the use of mass antibiotic distribution to potentially infected individuals.
Methods
Parameterization
Infectious Dose
There are 4 subspecies of F. tularensis, which have varying virulence; subspecies tularensis, also known as type A, causes the highest case fatality ratio and therefore raises the most concern as a potential bioweapon.2 Aerosol particle size is also known to have a significant impact on the infectivity and lethality for those individuals who have inhaled organisms.13,14 Here, and in later sections, we therefore focus on experiments using SCHU-S4 (a highly virulent type A strain) and evaluate published data that used particle diameters primarily in the 1-5-μm range (ie, the aerosol size generally considered capable of causing human/primate infection).
During the 1960s and 1970s, there were a number of published tularemia experiments performed on human volunteers.15–19 Jones et al7 recently combined a number of these data-sets and fitted Weibull and log-normal cumulative distribution functions to model the probability of infection given dose. These authors also considered the Saslaw et al16 results alone due to complications arising from pooling multiple data-sets.20 Here, we similarly restrict our analysis to the Saslaw et al results, since this is the only data-set to include multiple low-dose responses, which are important for our subsequent hazard assessments. Unlike Jones et al, we used the 1-parameter exponential model to fit the data, because it is the simplest relationship that can be derived from basic biological considerations20 such that:
 |
Eq. 1 |
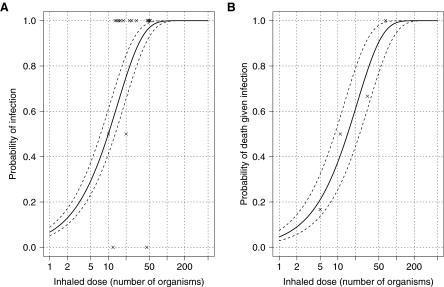
where p is the probability of infection given a dose d and rf is the probability that a single organism will initiate the infection. Figure 1A shows the data and exponential fit (rf=0.070, p-value=0.47 representing a good fit to the data) giving an ID50 (ie, the dose at which there is a 50% probability of infection) of ∼10 organisms with approximately 7% of individuals inhaling 1 organism likely being infected. The commonly used 2-parameter beta-Poisson and log-probit models20 did not provide sufficiently better fits to the data over the exponential model to justify the extra parameter. Primate data also support this high level of infectivity at low doses.13,18
Figure 1.
Dose-Response Figures of Inhalational Tularemia. (A) Solid and dashed lines represent best fit infectious dose-response and 95% confidence intervals, respectively, of the exponential model with parameter rf=0.070 based on human experimental data (crosses). (B) Solid and dashed lines represent best fit lethal dose-response and 95% confidence intervals, respectively, of the exponential model with parameter rd=0.047 based on primate experimental data (crosses).
Incubation Period
We identified 2 publications regarding vaccine trials in the literature that contained quantitative data relating to the human incubation period, each reporting on 16 infected volunteers.16,18 The earlier of these papers focused on low-dose experiments with prisoners, which resulted in 5- to 6-day incubation periods for the majority of nonvaccinated controls (×symbols, Figure 2A). The later study exposed soldiers to 25,000 organisms, which tended to cause symptoms after only 2 to 3 days (+symbols, Figure 2A). Following Wilkening's approach for estimating the dose-response to anthrax,21 a log-linear equation was fitted to these data:
 |
Eq. 2 |
Figure 2.
Incubation Period Figures of Inhalational Tularemia. (A) Solid and dashed lines represent best fit and 95% confidence bands, respectively, of the log-linear dose-dependent model (ic=−0.79, im=6.45) based on human experimental data (crosses). Dotted lines capture descriptive data not used in the fit due to a lack of information. (B) Dose-independent histogram using 531 cases experiencing various forms of tularemia.
where Ei is the incubation period in units of days, ic and im are the gradient and intercept parameters, respectively, and d is the inhaled dose. The solid line in Figure 2A shows the best fit to the data (ic=−0.79, im=6.45, R2=0.63). The dotted box in Figure 2A corresponds to the results of McCrumb, where, among the control subjects, clinically overt disease appeared within 3 to 5 days following exposure to a challenge varying from 200 to 20,000 organisms.15 In addition, Alluisi et al19 exposed 16 men to an aerosol containing 20,000 to 30,000 organisms and found that clinical illness began after incubation periods of 2 to 4 days. Similarly, Hornick and Eigelsbach found a mean incubation period of 3 days with volunteers exposed to 25,000 organisms.17 Although the latter 3 studies could not be incorporated explicitly into our analysis (because more precise individual-level data were not available), they are consistent with the log-linear fit.
Although vaccine experiments performed on human volunteers suggest that the incubation period is dose-dependent, there are also data from naturally acquired cases that can help to provide an insight into the epidemiology of tularemia. Figure 2B shows the incubation period of more than 500 such cases described by Foshay.22 It is important to note that these data include not only inhalational cases but also other forms of tularemia, including ulceroglandular and oculoglandular, which are likely to have resulted from infection through the skin and eyes, respectively. In addition, each case will have received a different dose, which is likely to have affected the incubation period. Despite these confounding effects, most of the cases have incubation periods of between 2 and 7 days, in agreement with the volunteer studies (note that standard probability distributions, such as log-normal, gamma, and Weibull, did not fit the data well). Table 1 shows some key measures of the incubation period, taken from a number of other tularemia studies.22–27 Across all of these studies, the average time from exposure to symptom onset varied between approximately 3 and 5 days, and the majority of cases showed symptoms within 2 weeks, though 3-week incubation periods were also observed. In addition, 3 further studies28–30 describing only cases of tularemia that experienced pulmonary involvement (ie, more likely to be inhalational cases) gave similar means and ranges to those described above (see Table 1).
Table 1.
Incubation Period Statistics
Time from Symptom Onset to Death
All of the human volunteer studies involved treatment following symptom onset; however, a literature search of primate experiments (see next section) revealed no evidence of dose-dependence with regard to the time from symptom onset to death. Therefore, we used data from naturally acquired cases of tularemia to provide a dose-independent symptom onset to death distribution. Since the most virulent type A strain occurs mainly in North America,1,31 the search for relevant cases was narrowed to literature from the U.S. Prior to the discovery of antibiotics such as streptomycin and the tetracyclines in the 1940s, it is perhaps unlikely that any other treatment significantly affected the symptomatic period, so we also focused on the pre–World War II era. Finally, the search was limited to those cases who were known to have died from pneumonia or pulmonary complications and, in particular, suffered the typhoidal form of tularemia that is likely to have resulted from inhalation.32–34
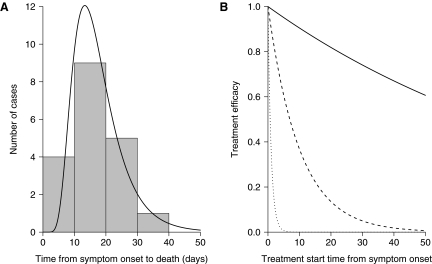
A log-normal distribution was fitted to the 19 known cases that met the above requirements (Figure 3A),22,23,28–30,35,36 giving a mean and standard deviation of 17.6 and 7.9 days, respectively. We confirmed the goodness of fit by performing a Cramer-Von-Mises normality test on the log-transformed data (p-value=0.33 representing a good fit to the data). The typhoidal form of tularemia can result from tick bites37 as well as infection by the assumed respiratory route. However, 8 of our 19 cases were exposed to rabbits, and the remaining 11 were either of unknown origin or not stated. Disturbing rabbit carcasses has been epidemiologically linked to inhalational tularemia,4,6,38,39 so it is possible that the source of infection for at least 8 of the cases was an aerosol from infected rabbit tissues. The average time to death for these cases was 16 days; thus, even if all the other cases (averaging 18.5 days) were infected via ticks, our best estimate of the time from symptom onset to death is still likely to remain approximately 2 weeks in duration for the majority of inhalational tularemia cases. Note that attempting to parameterize the time from symptom onset to recovery could be biased, since those cases with milder forms of the disease might be less likely to report to healthcare facilities and be documented.40
Figure 3.
Symptomatic Period Figures of Inhalational Tularemia. (A) Histogram and log-normal fit of the time from symptom onset until death for 19 naturally acquired untreated human cases (mean=17.6 days, s.d.=7.9 days). (B) Solid, dashed, and dotted lines represent assumed low (rt=0.01), medium (rt=0.1), and high (rt=1) rates of treatment decline, respectively, of the 1 minus exponential model.
Lethal Dose
Unlike almost all untreated anthrax41 and pneumonic plague42 cases, untreated tularemia is not consistently fatal. Primate experiments provide the best source of data to parameterize the probability of death given infection and dose, since human volunteers were always treated on becoming symptomatic. The data in Figure 1B are taken from Day and Berendt,13 where the survival of infected rhesus monkeys depended on their inhaled dose. Again, the exponential model (equation 1) provided a better fit to the data (rd=0.047, p-value=0.74 representing a good fit to the data) over the beta-Poisson and log-probit models. In close agreement, earlier experiments in the same laboratory found that 7 of 9 infected rhesus monkeys died after inhaling 35 organisms.43 Higher dose experiments consistently found 85% to 100% fatality rates for rhesus monkeys infected with doses between 170 and 10,000 organisms.43–45 However, pooling such studies did not improve on our original fit.
In a similar manner to the incubation period, although primate experiments show that the probability of surviving tularemia is dose-dependent, naturally acquired cases with pulmonary involvement can help inform the untreated human case fatality ratios for inhalational tularemia. Two such reviews of 26830 and 10024 cases both gave case fatality ratios of 40%, but 2 further studies of 95 and 51 cases quoted values of 30% and 80%, respectively.25,29 Smaller cohorts have similarly given case fatality ratios ranging from ∼10% to ∼60%.23,28,30,35 The fundamental problem with these estimates is that they likely include other noninhalational forms of tularemia. Interestingly, the studies that presented the 19 cases used to parameterize the symptom onset to death distribution also included 48 surviving typhoidal cases (with pulmonary involvement), giving an approximate 30% case fatality ratio. But as in the previous section, the case fatality estimates could be biased since those cases with milder forms of the disease might be less likely to report to healthcare facilities and be documented.
Treatment Efficacy
In a mass casualty situation, oral administration of doxycycline and ciprofloxacin would be the preferred treatment.1,2,46 Evans et al37 argued that prophylaxis is not recommended for tularemia because doxycycline is bacteriostatic and merely delays, rather than prevents, disease. However, earlier human volunteer studies have showed that infection with F. tularensis could be eradicated through bacteriostatic antibiotic therapy,18 and more recent experiments in mice have highlighted the effectiveness of prophylaxis with doxycycline and ciprofloxacin.47,48
In a review of tularemia therapy, Tarnvik and Chu concluded that “the period of treatment with a bacteriostatic agent needs to be long enough to allow development of a bactericidal host response.”34(p388) A review of naturally acquired tularemia cases showed a decline in treatment efficacy following a delay in starting treatment for both doxycycline and ciprofloxacin.49 Anecdotal evidence from a tularemia outbreak in Spain also found that delays in the diagnosis and initiation of therapy likely reduced the observed treatment success rates.50 Additionally, this positive relationship between treatment delay and failure has been noted by others,2,34,51 and experiments in mice similarly showed that protection decreased with increasing delays of initiating treatment.47 Interestingly, Sanders and Hahn found that “the duration of symptoms was usually shorter in those patients treated early,”26(p393) and likewise it has been suggested that “the longer the length of illness before therapy, the more likely that the response to treatment will be prolonged.”52(p267) Unfortunately, despite a wealth of qualitative information describing the effect of treatment on tularemia, there is little quantitative data available that could be used to suitably parameterize the impact of treatment in humans.
Modeling
We used atmospheric dispersion modeling to provide numbers and locations of symptomatic individuals that might be expected from an aerosolized release of F. tularensis. The U.S. Defense Threat Reduction Agency's Hazard Prediction and Assessment Capability (HPAC) uses SCIPUFF,53 a Lagrangian puff dispersion model. Although the version of HPAC that we used (4.0) does not include interference/complexities due to the urban terrain, our intention was to provide some general initial conditions for the subsequent public health response rather than a highly accurate picture of individual casualty locations. The HPAC software is able to calculate the potential dosages inhaled at predefined spatial coordinates. We assumed, therefore, that each individual situated in an administrative Great Britain ward when the dispersion cloud passed through would receive the same dose as an individual located at the ward centroid11 (population data were extracted from the 2001 Great Britain Census; see Appendix for further details: www.liebertonline.com/bsp). A fixed wind speed and direction were assumed for simplicity. Table 2 shows the parameters chosen to represent a relatively small release from the top of a building in London. (Given the resulting casualty numbers, the exact size of the release was deemed to be sensitive information and was therefore not included in Table 2.)
Table 2.
Release Parameters
Although the entire population of each affected ward was assumed to be exposed to F. tularensis, we also assumed that many individuals would be inside buildings shortly after the release and would, therefore, inhale lower doses than exposed individuals outside (see Table 2). The relationship described in Figure 1A was used to calculate the probability of infection for each individual exposed to F. tularensis given his or her inhaled dose. Infected individuals then passed through a log-normal incubation period with dose-dependent mean values provided by the log-linear fit in Figure 2A; we assumed the standard deviation to be fixed at 1. Each case was then attributed a dose-independent symptomatic period by sampling from the log-normal distribution shown in Figure 3A. The case fatality ratio for untreated symptomatic individuals followed the dose-response relationship in Figure 1B. Table 3 summarizes all disease parameters.
Table 3.
Disease Parameters
| Parameter | Description | Value |
|---|---|---|
| rf | Infectious dose | 0.070 |
| im | Incubation period intercept | 6.45 |
| ic | Incubation period gradient | –0.79 |
| Es | Symptomatic period mean | 17.6 days |
| SDs | Symptomatic period s.d. | 7.9 days |
| rd | Lethal dose | 0.047 |
Source for all information in this table is in this article.
Prophylactic treatment with antibiotics was assumed to be 100% effective in preventing symptom onset when administered during the incubation period.1 Given the lack of suitable quantitative data to model the effect of antibiotics being taken during the symptomatic period, 3 possible scenarios were considered that represent treatment efficacies under “low,” “medium,” and “high” rates of decline with time from symptom onset (the relationship is 1 minus the exponential model, or 1−equation 1; see Figure 3B). We investigated 2 intervention strategies: treating individuals as they became symptomatic (individual) and additional mass antibiotic distribution in wards where early symptomatic individuals were infected (collective). It was assumed that the hospital care system would be completely overburdened due to the large number of individuals seeking treatment,54 and it was therefore not included in our model.
We assumed that the release would occur over a short time period (of the order of minutes/hours) during the daytime, and thus individuals would be infected in the ward where they worked (“work” ward). The multivariate hypergeometric distribution was used to probabilistically allocate a “home” ward for each individual (commuting data were extracted from the 2001 Great Britain Census; see Appendix for further details) in order to show where symptomatic individuals might seek health care. Note that those individuals who do not work (or commute to a different ward) were assumed to stay in their home ward during the day. In the 1979 Sverdlovsk anthrax outbreak, the residences of many of the victims were scattered throughout the city, whereas their daytime locations on the estimated date of the release were mainly focused in a narrow band in the south of the city, where the dispersion cloud was projected to have passed through.55 For simplicity we therefore also assumed that public health workers would deduce a daytime release via early epidemiologic investigation and would therefore be able to implement the collective strategy for those working in the same ward as its initial case(s). We assumed that public health decision makers would be risk averse and also administer antibiotic courses to those individuals who lived (but didn't work) in wards identified for collective mass antibiotic distribution.
Prior to the implementation of either mitigation strategy it was assumed that there would be a minimum time delay of 2.5 or 4 days following the release to allow for outbreak detection. Under the individual strategy, we assumed that there would be a self-reporting lag of 1 day before symptomatic individuals received treatment. Additional mass antibiotic distribution under the collective strategy was assumed to occur over 2 or 6 further days following presentation of the first or tenth symptomatic individual. Based on the response to the 2001 anthrax attacks in the U.S., we assumed that 10% of individuals would not comply with the 14-day antibiotic course recommended for tularemia56 and would consequently follow an untreated disease progression. We also considered that a further 20% of individuals who had been potentially infected (and identified for prophylactic treatment under the collective strategy) might not comply with the full course of antibiotics compared with those suffering from disease symptoms. In addition, 1 in 10,000 of those survivors who completed the antibiotic course were assumed to suffer a serious adverse reaction to the treatment.57
Table 4 summarizes all (baseline and sensitivity analysis) intervention parameters. Since it is possible that the number of antibiotic courses available for responding to a large tularemia outbreak might be limited, we investigated how the optimal mitigation strategy was affected by stockpile levels of between 10,000 and 10 million antibiotic courses. To this end, we compared the individual and collective strategies in terms of which approach minimized the total number of deaths. Formally, the model used an individual-based stochastic framework with 100 simulations being performed for each scenario; a mathematical description is provided in the Appendix.
Table 4.
Intervention Parameters1
| Parameter | Description | Value | Reference |
|---|---|---|---|
| tg | Minimum time for outbreak detection | 2.5 (4) days | 54 |
| ts | Self-reporting delay following symptom onset | 1 day | This article |
| nt | Trigger number of symptomatic individuals in a ward for mass antibiotic distribution | 1 (10) | This article |
| td | Time to distribute antibiotics to an entire ward | 2 (6) days | 54 |
| c | Antibiotic compliance for symptomatic individuals | 0.9 | 54 |
| c | Antibiotic compliance for potentially infected individuals | 0.9 (0.7) | 64 |
| a | Antibiotic adverse event rate | 10−4 | 65 |
| rt | Treatment efficacy (1 minus exponential model) | 0.01 (0.1,0.001) | This article |
Values in parentheses were used for sensitivity analysis.
Results
Figure 4 inset gives an example of the numbers and work locations of those infected following the release described above in Methods. Individuals were infected up to 30 miles downwind of the source. The wards that were closer to the source, and in the path of the dispersion cloud, tended to have larger numbers of infected individuals since they generally received larger doses. The main map in Figure 4 indicates where these individuals were likely to live and therefore possibly report to local healthcare facilities. Although the wards enclosing the largest number of infected individuals had been directly affected by the dispersion cloud, infected individuals were likely to live throughout southeast England and were even scattered across the whole of Great Britain. In terms of the logistics of antibiotic distribution, it is worth briefly noting that approximately 70% and 30% of infected individuals lived further than 10 km and 20 km from work, respectively.
Figure 4.
Work (inset map) and Home (main map) Locations of Symptomatic Individuals. Modeled daytime aerosolized release of F. tularensis in central London (inset map). Multivariate hypergeometric redistribution of cases from work to home (main map). Ward boundaries source: 2001 Census, Output Area Boundaries. Crown copyright 2003. Crown copyright material is reproduced with the permission of the Controller of HMSO.
Averaging over all simulations, the total number of infected individuals was approximately 130,000 across 150 wards. Approximately 2.4 million individuals work (or live, for the nonworking population) in these wards, and thus at least this number of individuals were assumed to have been exposed to the agent. In the absence of treatment, the mean number of deaths was approximately 24,000, giving an overall case fatality ratio of about 18%. This value is within the limits of what might be expected given previously quoted case fatality ratios in the literature (see Methods) but is predicated on a relationship based on primate data (Figure 1B).
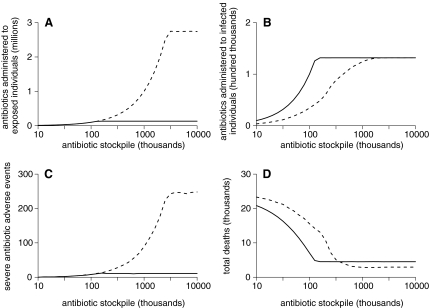
Figure 5A shows that, with the availability of a large antibiotic stockpile, more than 2.5 million more antibiotic courses were administered to those potentially infected with F. tularensis under the collective strategy compared to the individual strategy of treating only symptomatic individuals. With such a large stockpile, all infected individuals were captured by the collective strategy (see Figure 5B) but at the cost of approximately 250 severe antibiotic adverse events (see how Figure 5C essentially scales with Figure 5A). However, this number is relatively small compared with the ∼1,500 lives saved by providing early prophylactic treatment to asymptomatic individuals via the collective strategy (see Figure 5D).
Figure 5.
Baseline Results. Solid and dashed lines represent individual and collective strategies, respectively.
With lower stockpile levels, Figure 5A shows how the large number of antibiotic courses administered under the collective strategy is necessarily reduced with a subsequent reduction in the number of severe antibiotic adverse events (Figure 5C). There comes a point in Figure 5D where the individual strategy surpasses the collective strategy (in terms of fewer deaths) as the initial number of antibiotic courses available is lowered. Early mass antibiotic distribution is no longer beneficial, and those antibiotics that are “needlessly” used on uninfected individuals are much better spent on treating symptomatic individuals (contrast Figures 5A and 5B).
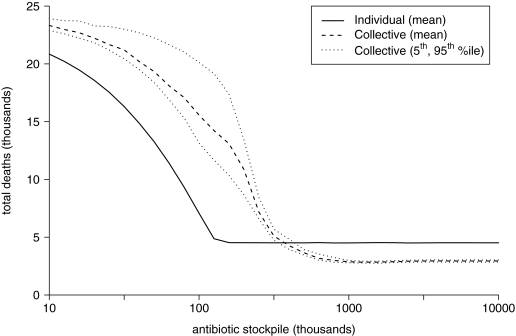
For the scenario described in the Methods, a collective strategy with a stockpile of approximately 1 million antibiotic courses is optimal in minimizing deaths, but a stockpile of 100,000 substantially favors the individual approach (Figure 5D). However, it is important to emphasize that such thresholds are not universal and will shift to lower and higher stockpiles with smaller and larger outbreaks, respectively (results not shown). Variability across simulations was only noteworthy for the number of deaths under the collective strategy with smaller stockpile levels (Figure 6), but this had little impact on the strategy threshold. Given that the size of the outbreak is perhaps the largest unknown, the remaining analysis focuses around sensitivity to intervention parameters over which public health authorities have some control.
Figure 6.
Uncertainty of Total Deaths over 100 Simulations
Figure 7A shows that if treatment is effective only in the very early stages following symptom onset and mass antibiotic distribution is rapid (2 days to complete), then the collective strategy might save more lives regardless of stockpile level. Alternatively, if treatment is still efficacious when begun well into the symptomatic period, then the individual strategy might be optimal for any given stockpile, even if mass antibiotic distribution is rapid (see Figure 7C). Indeed, the total deaths are far more sensitive to mass antibiotic distribution time (2 days vs. 6 days) when the treatment efficacy decline is assumed to be high rather than low (compare the difference between collective strategies in both Figure 7A and Figure 7C). Similar trends were found when, instead of increasing the time required for mass antibiotic distribution, outbreak detection time was increased from 2.5 days to 4 days (results not shown).
Figure 7.
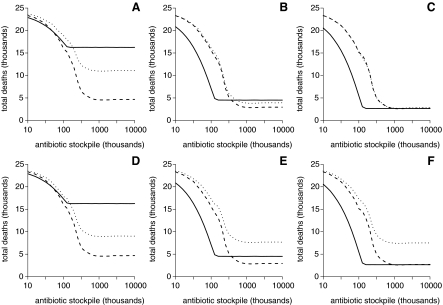
Sensitivity Analysis of Total Deaths when Varying the Speed of, and Compliance with, Mass Antibiotic Distribution. Solid and dashed lines show the average number of deaths for the individual and collective strategies, respectively, where treatment efficacies have high (A, D), medium (B, E), or low (C, F) rates of exponential decline. Dotted lines showing the average number of deaths for the collective strategy when antibiotic distribution takes 6 (instead of 2) days (A-C) and compliance with taking antibiotics is 70% (instead of 90%) (D-F).
Figures 7D and 7E show that if compliance with taking antibiotics in the collective strategy is 20% lower than in the individual strategy, then the benefit of mass antibiotic distribution at higher stockpile levels only remains so with a high rate of treatment efficacy decline. Unlike the time to initiate and complete mass antibiotic distribution, the effect of decreasing compliance with taking antibiotics was similar regardless of the treatment efficacy decline (compare the similarities of Figures 7A and 7D with the differences between 7C and 7F). Finally, we also found that increasing the number of symptomatic individuals in a ward from 1 to 10 before implementing mass antibiotic distribution in that ward made little difference to Figure 7 (results not shown). Since the adverse event rate was already set at a very conservative estimate and had already been shown to be an order of magnitude less than the total deaths (see Figure 5), this parameter was not varied in our sensitivity analysis.
Discussion
Our work began with a renewed review of the literature regarding key epidemiologic parameters, since the natural history of inhalational tularemia is relatively unknown, with earlier modeling studies reliant upon a number of assumptions.9,10 The infectious and lethal dose-response relationships, as well as the incubation period, were found to be dose-dependent based on analysis of human and primate experiments. The symptomatic period was based on the time from symptom onset until death for 19 naturally acquired cases of tularemia, suggesting an average 2-week window in which symptomatic individuals could potentially be recognized and treated, although the efficacy of treatment during this phase was difficult to quantify due to a lack of available data.
The second aspect of our study used atmospheric dispersion modeling software (HPAC) to assess the potential impact of a deliberate release of F. tularensis into a high-density civilian population. We showed that regular population movements in and around a Great Britain conurbation are likely to result in a wide spatial spread of infected individuals from the release when those infected at work return home. Many of these individuals would then be considerably outside of the original path of the dispersion cloud.
Finally, we considered 2 public health intervention strategies: treating only symptomatic individuals (individual) and the possible addition of mass antibiotic distribution to those who are potentially infected (collective). The individual strategy would be extremely dangerous for an outbreak of anthrax or pneumonic plague, because the similarly recommended prophylactic treatments of ciprofloxacin or doxycycline41,58 would likely prove ineffective beyond the first few days of symptoms. Indeed, our results have shown that a high rate of treatment efficacy decline for tularemia might favor the collective strategy regardless of stockpile level, especially if interventions are initiated and completed rapidly and compliance with taking antibiotics is high. However, evidence suggests that tularemia is more susceptible to delayed treatment than anthrax or pneumonic plague, giving weight to the individual strategy, especially for limited stockpiles or lower levels of compliance with taking antibiotics following a mass antibiotic distribution campaign.
A statistical method that estimates the location and geographic extent of a covert release of anthrax based on early case data (time of symptom onset and home/work locations) was recently published.11 Our parameterization of the incubation period and attack rate (infectious dose) of tularemia potentially also allow the application of this method following a release of F. tularensis. But if the fidelity of the spatial back-calculation model11 was deemed insufficient, then simply setting a non-zero number of symptomatic individuals in a specified area as the trigger to target that area, as described here, might be a sensible way to implement an alternative mass antibiotic distribution campaign. On the other hand, such an approach might be seen as a best-case alternative strategy, because only areas experiencing symptomatic individuals would receive antibiotics; if epidemiologic investigations proved particularly difficult, then it is possible that a wider area might have to be considered for mass antibiotic distribution due to a lack of detailed information of early symptomatic individuals' home or work locations. This would obviously require a larger antibiotic stockpile that would likely take additional time to distribute; in such circumstances, targeting individuals with symptoms of tularemia might be a better use of resources.
In the parameterization section of this study, we focused on the human (and primate) disease effects and accepted the default dispersion modeling parameters provided in the HPAC database, including the organism decay rate. However, future work could seek to review this parameter, analogous to recent anthrax-related research,59 especially given the number of publicly available documents relevant to the viability of F. tularensis in the atmosphere.60–63 Further, although solar radiation and other environmental factors would likely deactivate aerosolized organisms quite quickly (and secondary dispersal following an initial release is unlikely),1 wild and domestic animals could potentially be infected, leading to subsequent enzootic reservoirs of disease that could result in further human outbreaks.2 Infected animals might also act as sentinels following a deliberate pathogen release and offer an alternative method of estimating the exposed areas. Further research investigating such strategies might benefit from quantitative risk assessment and mathematical modeling.
It has previously been recommended that following a covert deliberate release of F. tularensis, those who are exposed should begin a “fever watch” and start treatment only after becoming symptomatic.1 Postexposure prophylaxis was recommended only in the event of an overt release where exposed individuals could be correctly identified. Here we have assessed these recommendations via a quantitative modeling study and have also addressed the question, “If antibiotics were in limited quantity, who would be the first to receive them?”10 We have found that with a sufficient stockpile of antibiotics that is dispensed rapidly following early outbreak detection, a targeted mass antibiotic distribution campaign with high levels of antibiotic compliance can prevent more deaths than treating symptomatic individuals alone. However, the longer treatment remains effective when started later into the symptomatic period and the longer it takes to initiate and complete mass antibiotic distribution, the more marginal the benefits of mass antibiotic distribution become. Targeting symptomatic individuals alone may even save more lives with limited antibiotic stockpile levels or with low levels of compliance following a mass antibiotic distribution campaign.
It is clear that the optimal strategy effectively depends on 2 competing timelines: on the one hand, the dose-dependent incubation period and the time-dependent efficacy of treatment and, on the other hand, the speed of detection of the outbreak, the speed of the distribution of countermeasures, and the degree of compliance by the public. Since these variables are based on limited data, and accurately estimating them is critical to realistic model output, it would seem that more research is called for. In particular, the duration of, and the treatment effectiveness within, the symptomatic period of tularemia is subject to significant uncertainty and would likely benefit from a similar detailed epidemiologic study to that performed for anthrax.41
Supplementary Material
Acknowledgments
Thanks to Emma Bennett, Judith Legrand, Neil Ferguson, Thomas House, Leon Danon, and Matt Keeling for helpful suggestions. This work was supported by the Department of Health for England (Health Protection Agency grant numbers 104307, 104308) and the Home Office (Office for Security and Counter-Terrorism grant number 17/05/70). The views and opinions expressed in this article are those of the authors and do not necessarily reflect those of the sponsoring institutions. The authors declare that they have no competing interests.
References
- 1.Dennis DT. Inglesby TV. Henderson DA, et al. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 2.Oyston PC. Sjostedt A. Titball RW. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat Rev Microbiol. 2004;2:967–978. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 3.Teutsch S. Martone W. Brink E. Pneumonic tularemia on Martha's Vineyard. N Engl J Med. 1979;301(15):826–828. doi: 10.1056/NEJM197910113011507. [DOI] [PubMed] [Google Scholar]
- 4.Feldman KA. Enscore RE. Lathrop SL, et al. An outbreak of primary pneumonic tularemia on Martha's Vineyard. N Engl J Med. 2001;345(22):1601–1606. doi: 10.1056/NEJMoa011374. [DOI] [PubMed] [Google Scholar]
- 5.Dahlstrand S. Ringertz O. Zetterberg B. Airborne tularemia in Sweden. Scand J Infect Dis. 1971;3(1):7–16. doi: 10.3109/inf.1971.3.issue-1.02. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy VP. Murphy MD. Lawnmower tularemia. Pediatr Infect Dis J. 1990;9(4):298–300. [PubMed] [Google Scholar]
- 7.Jones R. Nicas M. Hubbard A. Sylvester D. Reingold A. The infectious dose of Francisella tularensis (tularemia) Appl Biosaf. 2005;10(4):227–239. [Google Scholar]
- 8.U.S. Environmental Protection Agency; Office of Inspector General. Evaluation Report: EPA Needs to Fulfill Its Designated Responsibilities to Ensure Effective BioWatch Program. March 23, 2005. http://www.epa.gov/oig/reports/2005/20050323-2005-P-00012.pdf. [Aug 30;2011 ]. http://www.epa.gov/oig/reports/2005/20050323-2005-P-00012.pdf
- 9.Kaufmann AF. Meltzer MI. Schmid GP. The economic impact of a bioterrorist attack: are prevention and postattack intervention programs justifiable? Emerg Infect Dis. 1997;3(2):83–94. doi: 10.3201/eid0302.970201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Health Aspects of Chemical and Biological Weapons. Geneva: World Health Organization; 1970. [Google Scholar]
- 11.Legrand J. Egan JR. Hall IM. Cauchemez S. Leach S. Ferguson NM. Estimating the location and spatial extent of a covert anthrax release. PLoS Comput Biol. 2009 Jan;5(1):e1000356. doi: 10.1371/journal.pcbi.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egan JR. Legrand J. Hall IM. Cauchemez S. Ferguson NM. Leach S. Re-assessment of mitigation strategies for deliberate releases of anthrax using a real-time outbreak characterization tool. Epidemics. 2010;2(4):189–194. doi: 10.1016/j.epidem.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Day WC. Berendt RF. Experimental tularemia in Macaca mulatta: relationship of aerosol particle size to the infectivity of airborne Pasteurella tularensis. Infect Immun. 1972;5(1):77–82. doi: 10.1128/iai.5.1.77-82.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen JS. Concepts of offensive use of biological weapons. Militaert Tidsskrift. 2006;135:347–359. [Google Scholar]
- 15.McCrumb FR. Aerosol infection of man with Pasteurella tularensis. Bacteriol Rev. 1961;25(3):262–267. doi: 10.1128/br.25.3.262-267.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saslaw S. Eigelsbach HT. Prior JA. Wilson HE. Carhart S. Tularemia vaccine study. II. Respiratory challenge. Arch Intern Med. 1961;107:702–714. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- 17.Hornick RB. Eigelsbach HT. Aerogenic immunization of man with live tularemia vaccine. Bacteriol Rev. 1966;30(3):532–538. doi: 10.1128/br.30.3.532-538.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawyer WD. Dangerfield HG. Hogge AL. Crozier D. Antibiotic prophylaxis and therapy of airbourne tularemia. Bacteriol Rev. 1966;30(3):542–550. doi: 10.1128/br.30.3.542-550.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alluisi EA. Beisel WR. Bartelloni PJ. Behavioral effects of tularemia and sandfly fever in man. J Infect Dis. 1973;128(6):710–717. doi: 10.1093/infdis/128.6.710. [DOI] [PubMed] [Google Scholar]
- 20.Bartrand T. Weir M. Haas C. Dose-response models for inhalation of Bacillus anthracis spores: interspecies comparisons. Risk Anal. 2008;28(4):1115–1124. doi: 10.1111/j.1539-6924.2008.01067.x. [DOI] [PubMed] [Google Scholar]
- 21.Wilkening DA. Sverdlovsk revisited: modeling human inhalation anthrax. Proc Natl Acad Sci U S A. 2006;103(20):7589–7594. doi: 10.1073/pnas.0509551103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foshay L. Tularemia: a summary of certain aspects of the disease including methods for early diagnosis and the results of serum treatment in 600 patients. Medicine. 1940;19:1–83. [Google Scholar]
- 23.Kavanaugh CN. Tularemia: a consideration of one hundred and twenty-three cases with observations at autopsy in one. Arch Intern Med. 1935;55:61–85. [Google Scholar]
- 24.Cecil RL. Textbook of Medicine. Philadelphia and London: W.B. Saunders; 1938. [Google Scholar]
- 25.Dienst FT., Jr Tularemia: a perusal of three hundred thirty-nine cases. J La State Med Soc. 1963;115:114–127. [PubMed] [Google Scholar]
- 26.Sanders CV. Hahn R. Analysis of 106 cases of tularaemia. J La State Med Soc. 1968;120(9):391–393. [PubMed] [Google Scholar]
- 27.Pullen RL. Stuart BM. Tularaemia: analysis of 235 cases. JAMA. 1945;129:495–500. [Google Scholar]
- 28.Blackford SD. Pulmonary manifestations in human tularemia: a clinical study based on thirty-five unselected cases. JAMA. 1935;104(11):891–895. [Google Scholar]
- 29.Blackford SD. Casey CJ. Pleuropulmonary tularemia. Arch Intern Med. 1941;67:43–71. [Google Scholar]
- 30.Stuart BM. Pullen RL. Tularemic pneumonia; review of American literature and report of 15 additional cases. Am J Med Sci. 1945;210:223–236. [Google Scholar]
- 31.Bossi P. Tegnell A. Baka A, et al. BICHAT guidelines for the clinical management of tularaemia and bioterrorism-related tularaemia. Euro Surveill. 2004;9(12):9–10. doi: 10.2807/esm.09.12.00503-en. [DOI] [PubMed] [Google Scholar]
- 32.Avery F. Barnett T. Pulmonary tularemia. A report of five cases and consideration of pathogenesis and terminology. Am Rev Respir Dis. 1967;95(4):584–591. doi: 10.1164/arrd.1967.95.4.584. [DOI] [PubMed] [Google Scholar]
- 33.Franz DR. Jahrling PB. Friendlander AM, et al. Clinical recognition and management of patients exposed to biological warfare agents. JAMA. 1997;278(5):399–411. doi: 10.1001/jama.278.5.399. [DOI] [PubMed] [Google Scholar]
- 34.Tärnvik A. Chu MC. New approaches to diagnosis and therapy of tularemia. Ann N Y Acad Sci. 2007;1105:378–404. doi: 10.1196/annals.1409.017. [DOI] [PubMed] [Google Scholar]
- 35.Bihss T. Berland H. Roentgenological manifestations of pleuro-pulmonary involvement in tularemia. Radiology. 1943;431:431–437. [Google Scholar]
- 36.Permar H. Maclachlan W. Tularemic pneumonia. Ann Intern Med. 1931;5:687–698. [Google Scholar]
- 37.Evans ME. Gregory DW. Schaffner W. McGee ZA. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore) 1985;64(4):251–269. [PubMed] [Google Scholar]
- 38.Martone WJ. Marshall LW. Kaufmann AF. Hobbs JH. Levy ME. Tularemia pneumonia in Washington, DC. A report of three cases with possible common-source exposures. JAMA. 1979;242(21):2315–2317. doi: 10.1001/jama.242.21.2315. [DOI] [PubMed] [Google Scholar]
- 39.Halsted CC. Kulasinghe HP. Tularemia pneumonia in urban children. Pediatrics. 1978;61(4):660–662. [PubMed] [Google Scholar]
- 40.Garske T. Legrand J. Donnelly CA, et al. Assessing the severity of the novel influenza A/H1N1 pandemic. BMJ. 2009;339(7714):220–224. doi: 10.1136/bmj.b2840. [DOI] [PubMed] [Google Scholar]
- 41.Holty JE. Bravata DM. Liu H. Olshen RA. McDonald KM. Owens DK. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med. 2006;144(4):270–280. doi: 10.7326/0003-4819-144-4-200602210-00009. [DOI] [PubMed] [Google Scholar]
- 42.Egan JR. A plague on five of your houses—statistical re-assessment of three pneumonic plague outbreaks that occurred in Suffolk, England, between 1906 and 1918. Theor Biol Med Model. 2010;7:39. doi: 10.1186/1742-4682-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodlow RJ. Leonard FA. Viability and infectivity of microorganisms in experimental airborne infection. Bacteriol Rev. 1961;25:182–187. doi: 10.1128/br.25.3.182-187.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eigelsbach HT. Tulis JJ. Overholt EL. Griffith WR. Aerogenic immunization of the monkey and guinea pig with live tularemia vaccine. Proc Soc Exp Biol Med. 1961;108:732–734. doi: 10.3181/00379727-108-27049. [DOI] [PubMed] [Google Scholar]
- 45.Tulis JJ. Eigelsbach HT. Hornick RB. Oral vaccination against tularemia in the monkeys. Proc Soc Exp Biol Med. 1969;132(3):893–897. doi: 10.3181/00379727-132-34331. [DOI] [PubMed] [Google Scholar]
- 46.Health Protection Agency. Guidelines for Action in the Event of a Deliberate Release: Tularemia. www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1194947357555. [Sep 1;2011 ]. www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1194947357555
- 47.Russell P. Eley SM. Fulop MJ. Bell DL. Titball RW. The efficacy of ciprofloxacin and doxycycline against experimental tularaemia. J Antimicrob Chemother. 1998;41(4):461–465. doi: 10.1093/jac/41.4.461. [DOI] [PubMed] [Google Scholar]
- 48.Piercy T. Steward J. Lever MS. Brooks TJ. In vivo efficacy of fluoroquinolones against systemic tularaemia infection in mice. J Antimicrob Chemother. 2005;56(6):1069–1073. doi: 10.1093/jac/dki359. [DOI] [PubMed] [Google Scholar]
- 49.Enderlin G. Morales L. Jacobs RF. Cross JT. Streptomycin and alternative agents for the treatment of tularemia: review of the literature. Clin Infect Dis. 1994;19(1):42–47. doi: 10.1093/clinids/19.1.42. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Castrillon JL. Bachiller-Luque P. Martin-Luquero M. Mena-Martin FJ. Herreros V. Tularemia epidemic in northwestern Spain: clinical description and therapeutic response. Clin Infect Dis. 2001;33(4):573–576. doi: 10.1086/322601. [DOI] [PubMed] [Google Scholar]
- 51.Tärnvik A. Berglund L. Tularaemia. Eur Respir J. 2003;21(2):361–373. doi: 10.1183/09031936.03.00088903. [DOI] [PubMed] [Google Scholar]
- 52.Penn RL. Kinasewitz GT. Factors associated with a poor outcome in tularaemia. Arch Intern Med. 1987;147:265–268. [PubMed] [Google Scholar]
- 53.Sykes R. Parker S. Henn D. Chowdhury B. SCIPUFF Version 2.3, Technical Documentation. Apr, 2007.
- 54.Wein LM. Craft DL. Kaplan EH. Emergency response to an anthrax attack. Proc Natl Acad Sci U S A. 2003;100(7):4346–4351. doi: 10.1073/pnas.0636861100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guillemin J. Anthrax: The Investigation of a Deadly Outbreak. Berkeley: University of California Press; 2001. [Google Scholar]
- 56.Shepard CW. Soriano-Gabarro M. Zell ER, et al. Antimicrobial postexposure prophylaxis for anthrax: adverse events and adherence. Emerg Infect Dis. 2002;8(10):1124–1132. doi: 10.3201/eid0810.020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meropol SB. Chan KA. Chen Z, et al. Adverse events associated with prolonged antibiotic use. Pharmacoepidemiol Drug Saf. 2008;17(5):523–532. doi: 10.1002/pds.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inglesby TV. Dennis DT. Henderson DA, et al. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 2000;283(17):2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- 59.Stuart AL. Wilkening DA. Degradation of biological weapons agents in the environment: implications for terrorism response. Environ Sci Technol. 2005;39(8):2736–2743. doi: 10.1021/es048705e. [DOI] [PubMed] [Google Scholar]
- 60.Hood AM. Infectivity of Pasteurella tularensis clouds. J Hyg (Lond) 1961 Dec;59:497–504. doi: 10.1017/s002217240003919x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goodlow RJ. Leonard FA. Viability and infectivity of microorganisms in experimental airborne infection. Bacteriol Rev. 1961 Sep;25:182–187. doi: 10.1128/br.25.3.182-187.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sawyer WD. Jemski JV. Hogge AL, Jr, et al. Effect of aerosol age on the infectivity of airborne Pasteurella tularensis for Macaca mulatta and man. J Bacteriol. 1966;91(6):2180–2184. doi: 10.1128/jb.91.6.2180-2184.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cox CS. Aerosol survival of Pasteurella tularensis disseminated from the wet and dry states. Appl Microbiol. 1971;21(3):482–486. doi: 10.1128/am.21.3.482-486.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wein LM. Craft DL. Evaluation of public health interventions for anthrax: a report to the Secretary's Council on Public Health Preparedness. Biosecur Bioterror. 2005;3:348–356. doi: 10.1089/bsp.2005.3.348. [DOI] [PubMed] [Google Scholar]
- 65.Fowler RA. Sanders GD. Bravata DM, et al. Cost-effectiveness of defending against bioterrorism: a comparison of vaccination and antibiotic prophylaxis against anthrax. Ann Intern Med. 2005;142:601–610. doi: 10.7326/0003-4819-142-8-200504190-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.