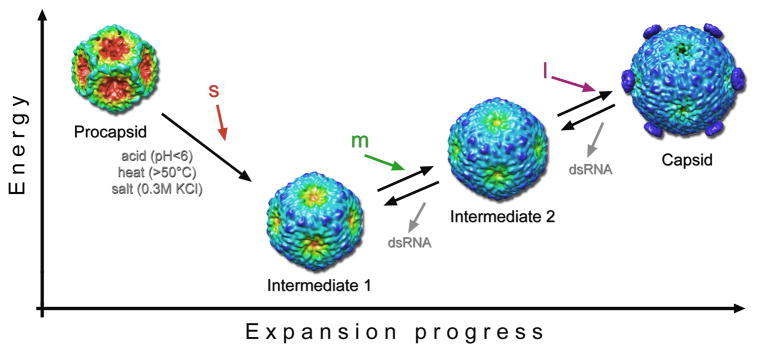
Figure 6.
Sequential steps of procapsid expansion. Here we tentatively correlate the structures produced by perturbing the procapsid or the RNA-filled capsid in vitro by environmental factors (acid, heat and/or salt) with packaging intermediates that it has not yet been possible to observe directly. The procapsid transforms in a cooperative manner to the intermediate 1 state in vitro in response to any of several treatments or, we hypothesize, by packaging the s segment. Further transformation to the intermediate 2 state would then be driven by packaging the m segment. Finally, packaging of the l segment and RNA replication yield the fully expanded capsid. To date, intermediate 2 has only been observed on perturbing the mature capsid and not on driving expansion further past the intermediate 1 state. This observation suggests that the lowest free energy state of the P1 shell in the absence of packaged RNA is intermediate 1. The reconstructions are color-coded according to the radial distance from the particle center from red to blue to emphasize the main conformational change: the outward movement of the five-fold vertices. Vertex-mounted P4 hexamers are present on the capsid reconstruction and are inferred to have been shed from the particles used to calculate the intermediate 1 and 2 reconstructions (see Discussion).