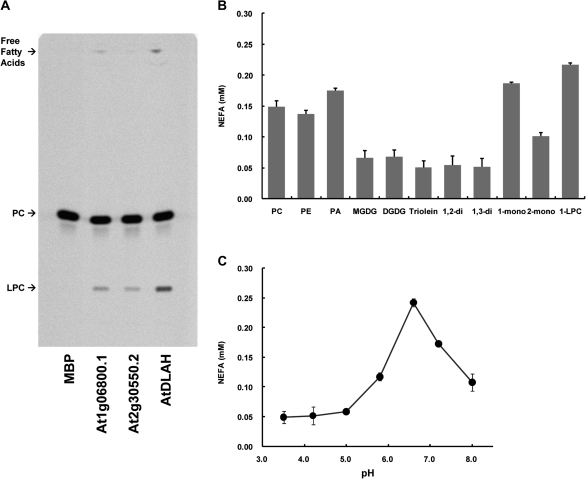
Fig. 2.
AtDLAH enzyme assays. (A) AtDLAH catalyses the hydrolysis of PC at the sn-1 position. PLA1 activity was measured by the production of radiolabelled lysophosphatidylcholine (LPC) after incubation with 1-palmitoyl-2-[14C]palmitoyl-PC. The resultant 14C-labelled LPC was detected by TLC. MBP was used as a negative control, and At1g06800.1 and At2g30550.2, which have PLA1 activity (Seo et al., 2009), were used as positive controls. (B) Lipolytic enzyme assays to determine substrate specificity. Lipolytic activities were determined using an NEFA-HR kit with PC, PE, PA, MGDG, DGDG, triolein, 1,2-diacylglycerol, 1,3-diacylglycerol, 1-monodiacylglycerol, 2-monodiacylglycerol, and 1-LPC as substrates. Results are expressed as the means ±SD from four independent experiments. (C) Optimal pH for AtDLAH activity. Lipase activity of AtDLAH was determined by quantifying the release of free fatty acids from 1-LPC in phosphate buffers with different pHs at 30 °C for 30 min. Results are expressed as the means ±SD from four independent experiments.