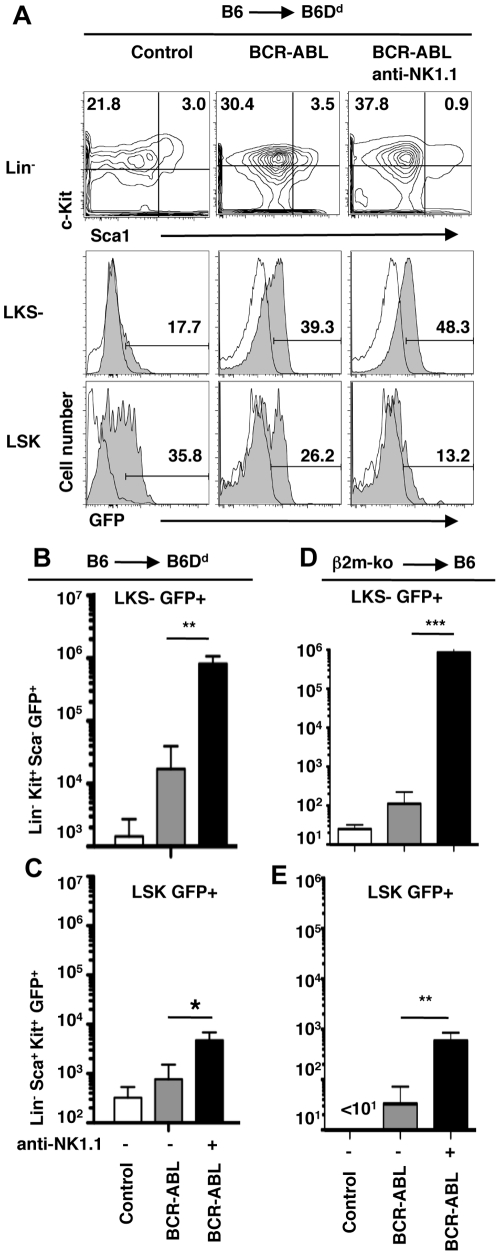
Figure 6. NK cell mediated missing-self recognition reduces the abundance of BCR-ABL1+ leukemia initiating cells.
(A) B6 BM cells (H-2b) were transduced with a retrovirus expressing GFP (Control) or BCR-ABL1 plus GFP (BCR-ABL1) and transplanted into lethally irradiated B6Dd recipient mice (H-2bDd). Recipient spleens were analyzed by flow cytometry at d8 post transplantation. Donor-derived cells lacking markers of mature lineage cells (Lin-) were analyzed for the expression of c-kit and Sca-1 (top row). Histograms (gray filled) depict GFP expression (Control or BCR-ABL1) in gated Lin- c-kit+ sca-1- (LKS-) cells (myeloid/erythroid progenitors) (middle row) and in Lin- c-kit+ sca-1+ (LSK) cells (haematopoietic stem cell compartment) (bottom row). Open histograms depict background staining using BM precursor cells from normal B6 mice. Numbers indicate the percentage of cells in the respective gate. Some recipient mice had been depleted of NK1.1+ cells (anti-NK1.1). The bar graphs show the mean absolute number (±SD) of donor-derived GFP+ (Control or BCR-ABL1) LKS- cells (myeloid/erythroid progenitors) (B) and LSK cells (haematopoietic stem cell compartment) (C) at d8 after transplantation. (D, E) The bar graphs show the mean absolute number (±SD) of β2m-ko-derived GFP+ (Control or BCR-ABL1) LKS- cells (D) and LSK cells (E) at d8 after transplantation into B6 (H-2b) recipients. Significant differences between groups are indicated as * p<0.05, ** p<0.01 and *** p<0.001; (ns) not significantly different p>0.05.