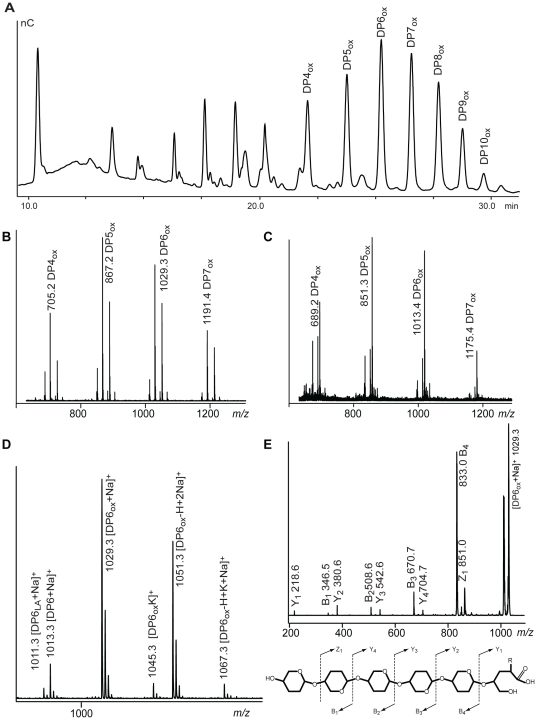
Figure 3. Products generated from cellulose by PcGH61D.
Panel A shows a typical HPAEC chromatogram of products obtained upon incubation of 0.1% PASC with 40 µg/mL PcGH61D in 25mM Tris (not MES which is not good for MALDI) pH 6.5, 1mM ascorbic acid (not reduced glutathione which is not good for MALDI), overnight at 50°C. The chromatogram shows a range of oxidized oligosaccharides (DP 4-10) as well as native oligosaccharides (for information on chromatographic standards, see Forsberg et al. [7]). Panel B shows the MALDI spectrum of the same sample as in Panel A focusing on the most prominent masses in the spectrum. All ion clusters for oxidized products had a similar distribution, the most abundant peak being the Na-adduct of the aldonic acid, which is annotated with DPnox and its m/z. Panel C displays the MALDI-TOF-mass spectrum of the same sample as in panel B after saturation with lithium (20 mM) to obtain lithium adducts only. This was done to eliminate the possibility that the compound annotated as the aldonic acid in fact was a K-adduct of the native oligosaccharide (which would give the same mass as the Na-adduct of the oxidized oligosaccharide). Indeed Li-adducts ([M+Li]) and the corresponding lithium salt of the lithium adduct [M+2Li-H]) occur in pairs throughout the spectrum replacing completely the more complex cluster of Na- and K-adducts/salts (m/z -16 relative to the sodium adducts of panel B; only the lithium adducts of the aldonic acids are annotated). This confirms the presence of aldonic acids. For further clarification a detailed view of the ion cluster for DP6ox (Glc5GlcA) from Panel B is shown in Panel D. The cluster contains the Na-adducts of the gluconolacton (DP6La, m/z 1011), cellohexaose (DP6, m/z 1013) and oxidized cellohexaose (DP6ox; m/z 1029). In addition, the spectrum shows the Na-salt of the Na-adduct of DP6ox (m/z 1051), the K-adduct of DP6ox (m/z 1045) and the Na-salt of the K-adduct of DP6ox (m/z 1067). To verify the presence and position of the acid group, MS2 experiments were done for several ions. Panel E displays fragments obtained for DP6ox (m/z 1029) with fragment ions named according to the Domon and Costello nomenclature [34]. The spectrum corresponds to that of a cello-oligosaccharide with an aldonic acid in the reducing end, and repeating hexose units towards the non-reducing end. The nomenclature used for products throughout this report is: DPn, cello-oligosaccharide with n glucose residues; DPnox, cello-oligosaccharides with n-1 glucose residues + one gluconic acid [Glc(n-1)GlcA]; DPnLA, cello-oligosaccharides with n-1 glucose residues + one gluconolactone [Glc(n-1)GlcLA].