Abstract
Weanling rats were fed diets which contained either no protein or 27% protein. In one experiment after 23-35 days both groups were given l-leucine-4,5-3H either intragastrically or intraperitoneally and then sacrificed 24 hr later. In a second experiment animals were given these diets for 21 days and sacrificed 3, 6, or 12 hr after either intragastric or intraperitoneal administration of the labeled leucine. In both experiments the intestinal mucosa of proximal and distal segments of the small intestine was scraped, weighed, the protein concentration measured, and the specific activty of the mucosal protein was determined.
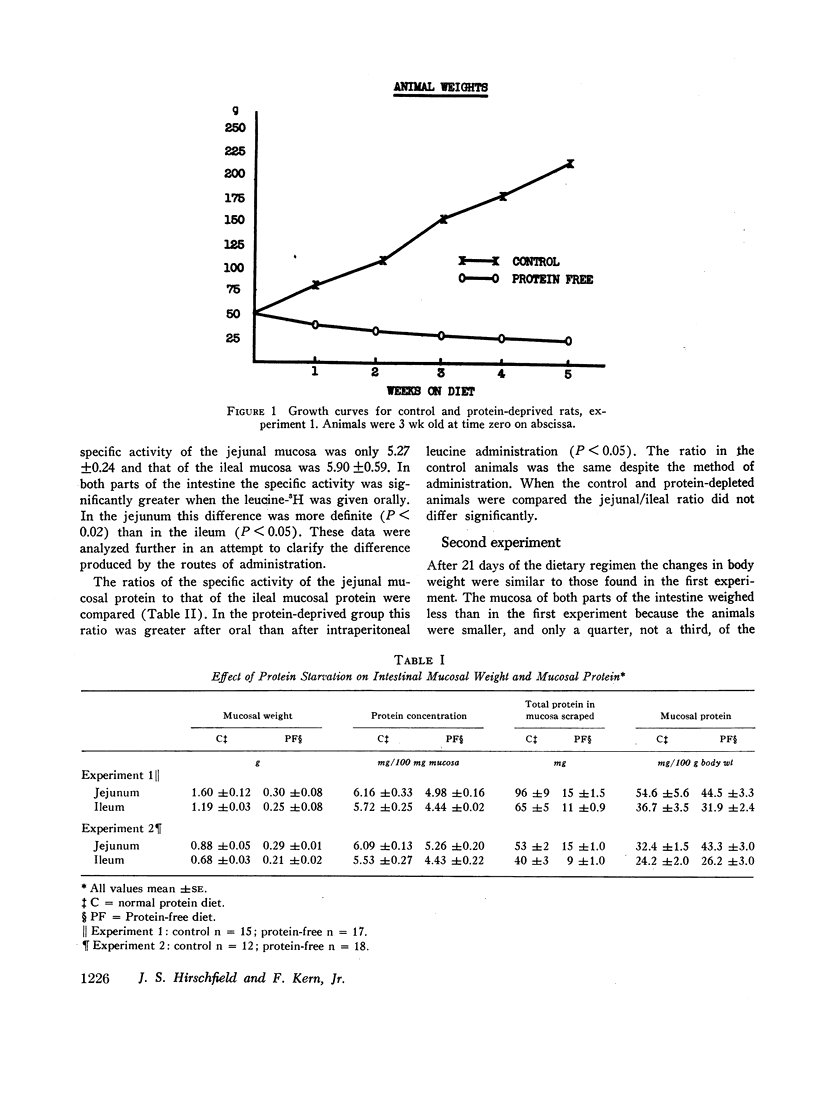
The wet weight of the mucosa and the protein concentration of the mucosa were significantly greater in the control animals than in the protein-depleted animals. The mucosal protein per 100 g of body weight was the same in the protein-deprived and the control groups. The specific activity of the intestinal mucosal protein was higer in the protein-deprived animals than in the control animals.
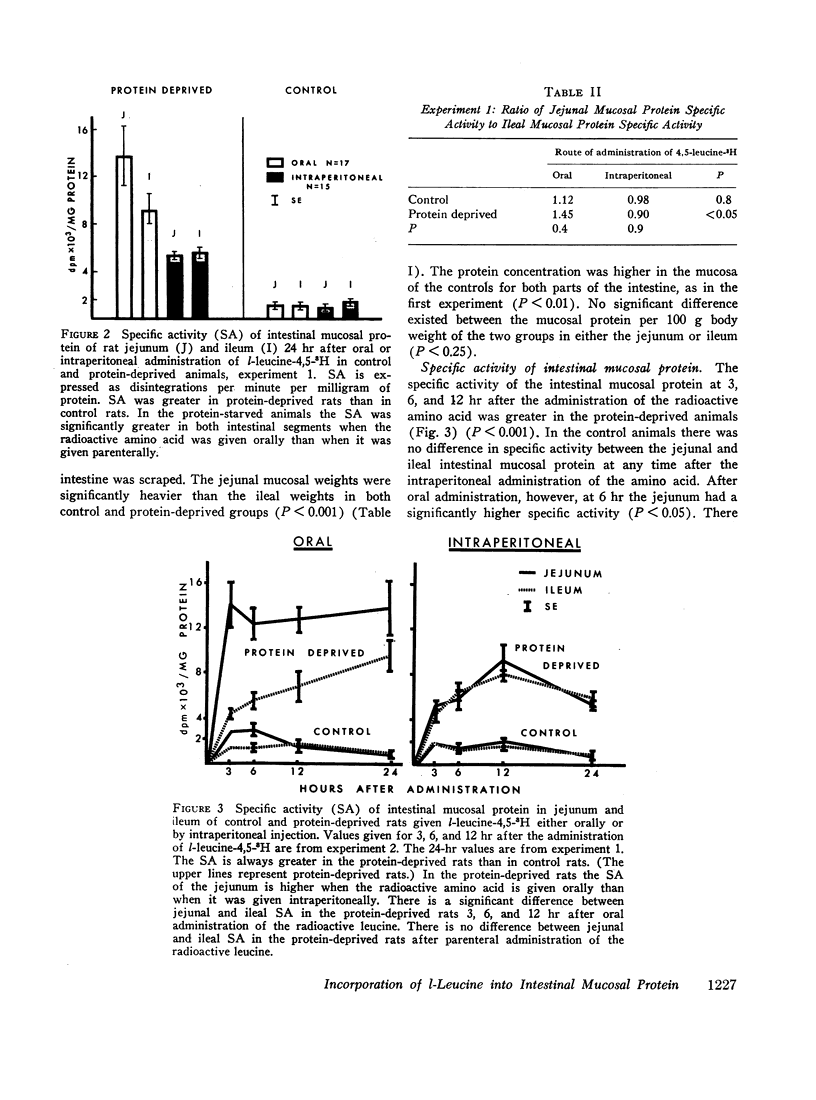
In the protein-deprived animals the proximal segment incorporated more radioactive amino acid into mucosal protein than did the distal segment at 3, 6, 12, and 24 hr after the amino acid was given by mouth. A similar difference was found between the proximal and distal segments of the control animals 6 hr after oral adminisstration of l-leucine-3H. On the other hand, when the l-leucine-3H was given intraperitoneally to both groups of animals there was no difference between proximal and distal small intestine. These findings suggest that intestinal mucosal protein can be synthesized directly from intraluminal amino acids, especially during protein deprivation, and that endogenous intraluminal protein might be important in the nutrition of the small intestinal mucosa.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS C. W., FERNAND V. S., SCHNIEDEN H. Histochemistry of a condition resembling kwashiorkor produced in rodents by a low protein-high carbohydrate diet (Cassava). Br J Exp Pathol. 1958 Aug;39(4):393–404. [PMC free article] [PubMed] [Google Scholar]
- DAWSON R., HOLDSWORTH E. S. An investigation into protein digestion with 14C-labelled protein. 1. The general pattern of 14C incorporation in body tissues and fluids of the rat up to 3 h after feeding. Br J Nutr. 1962;16:13–25. doi: 10.1079/bjn19620002. [DOI] [PubMed] [Google Scholar]
- DEO M. G., RAMALINGASWAMI V. ABSORPTION OF CO58 LABELED CYANOCOBALAMIN IN PROTEIN DEFICIENCY. AN EXPERIMENTAL STUDY IN THE RHESUS MONKEY. Gastroenterology. 1964 Feb;46:167–174. [PubMed] [Google Scholar]
- DEO M. G., RAMALINGASWAMI V. REACTION OF THE SMALL INTESTINE TO INDUCED PROTEIN MALNUTRITION IN RHESUS MONKEYS. A STUDY OF CELL POPULATION KINETICS IN THE JEJUNUM. Gastroenterology. 1965 Aug;49:150–157. [PubMed] [Google Scholar]
- HOOPER C. S., BLAIR M. The effect of starvation on epithelial renewal in the rat duodenum. Exp Cell Res. 1958 Feb;14(1):175–181. doi: 10.1016/0014-4827(58)90224-6. [DOI] [PubMed] [Google Scholar]
- Hill R. B., Jr, Prosper J., Hirschfield J. S., Kern F., Jr Protein starvation and the small intestine. I. The growth and morphology of the small intestine in weanling rats. Exp Mol Pathol. 1968 Feb;8(1):66–74. doi: 10.1016/0014-4800(68)90006-3. [DOI] [PubMed] [Google Scholar]
- JU J. S., NASSET E. S. Changes in total nitrogen content of some abdominal viscera in fasting and realimentation. J Nutr. 1959 Aug 10;68(4):633–645. doi: 10.1093/jn/68.4.633. [DOI] [PubMed] [Google Scholar]
- KERSHAW T. G., NEAME K. D., WISEMAN G. The effect of semistarvation on absorption by the rat small intestine in vitro and in vivo. J Physiol. 1960 Jun;152:182–190. doi: 10.1113/jphysiol.1960.sp006480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBLOND C. P., EVERETT N. B., SIMMONS B. Sites of protein synthesis as shown by radioautography after administration of S35-labelled methionine. Am J Anat. 1957 Sep;101(2):225–271. doi: 10.1002/aja.1001010203. [DOI] [PubMed] [Google Scholar]
- LIPKIN M., QUASTLER H. Studies of protein metabolism in intestinal epithelial cells. J Clin Invest. 1962 Mar;41:646–653. doi: 10.1172/JCI104520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MURAMATSU K., SATO T., ASHIDA K. DIETARY PROTEIN LEVEL AND THE TURNOVER RATE OF TISSUE PROTEINS IN RATS. J Nutr. 1963 Dec;81:427–433. doi: 10.1093/jn/81.4.427. [DOI] [PubMed] [Google Scholar]
- NASSET E. S., SCHWARTZ P., WEISS H. V. The digestion of proteins in vivo. J Nutr. 1955 May 10;56(1):83–94. doi: 10.1093/jn/56.1.83. [DOI] [PubMed] [Google Scholar]
- Nasset E. S. Role of the digestive system in protein metabolism. Fed Proc. 1965 Jul-Aug;24(4):953–958. [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- PRICE R. W. Effects of dietary protein level and starvation on the mucosal surface of the small intestine. Aerosp Med. 1962 Jan;33:42–49. [PubMed] [Google Scholar]
- Prosper J., Murray R. L., Kern F., Jr Protein starvation and the small intestine. II. Disaccharidase activities. Gastroenterology. 1968 Aug;55(2):223–228. [PubMed] [Google Scholar]
- SCHREIER K., KAZASSIS C. Influence of different types of malnutrition on the 14C-lysine metabolism in young rats. Nature. 1960 Sep 24;187:1117–1118. doi: 10.1038/1871117a0. [DOI] [PubMed] [Google Scholar]
- SCRIMSHAW N. S., BEHAR M., PEREZ C., VITERI F. Nutritional problems of children in Central America and Panama. Pediatrics. 1955 Sep;16(3):378–397. [PubMed] [Google Scholar]
- SHORTER R. G., CREAMER B. Ribonucleic acid and protein metabolism in the gut. I. Observations in gastro-intestinal cells with rapid turnover. Gut. 1962 Jun;3:118–128. doi: 10.1136/gut.3.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENRAM U. Radioautographic studies with methionine-3H and cytidine-3H on protein deficiency in mice and rats with special reference to liver cells. Exp Cell Res. 1962 Mar;26:485–492. doi: 10.1016/0014-4827(62)90153-2. [DOI] [PubMed] [Google Scholar]
- Saunders S. J., Isselbacher K. J. Inhibition of intestinal amino acid transport by hexoses. Biochim Biophys Acta. 1965 Jul 22;102(2):397–409. doi: 10.1016/0926-6585(65)90130-5. [DOI] [PubMed] [Google Scholar]
- TAKANO J. INTESTINAL CHANGES IN PROTEIN-DEFICIENT RATS. Exp Mol Pathol. 1964 Jun;86:224–231. doi: 10.1016/0014-4800(64)90055-3. [DOI] [PubMed] [Google Scholar]
- TYE R., ENGEL J. D. LIQUID SCINTILLATION COUNTING OF CARBON-14 IN AQUEOUS DIGESTS OF WHOLE TISSUES. Anal Chem. 1965 Sep;37:1225–1227. doi: 10.1021/ac60229a013. [DOI] [PubMed] [Google Scholar]
- Tandon B. N., Magotra M. L., Saraya A. K., Ramalingaswami V. Small intestine in protein malnutrition. Am J Clin Nutr. 1968 Aug;21(8):813–819. doi: 10.1093/ajcn/21.8.813. [DOI] [PubMed] [Google Scholar]
- WATERLOW J. Effect of protein depletion on the distribution of protein synthesis. Nature. 1959 Dec 12;184(Suppl 24):1875–1876. doi: 10.1038/1841875a0. [DOI] [PubMed] [Google Scholar]


