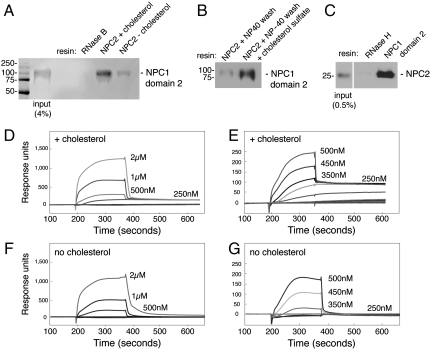
Fig. 3.
NPC2 binding to NPC1 lumenal domain 2 requires cholesterol. (A) Immunoblot of NPC1 domain 2 binding to NHS-Sepharose to which either RNase B or NPC2 (2 mg/mL resin) has been covalently attached. NPC1 domain 2 (2 μM) was added for 30 min at 30 °C; for reactions with or without cholesterol (as indicated), samples received either cholesterol sulfate (60 μM) or 0.1% Nonidet P-40 for 10 min, followed by a wash with 10 volumes 50 mM MES (pH 5.5), 150 mM NaCl. Elution was with pH 8.0 buffer. (B) Binding of NPC1 domain 2 to immobilized NPC2 was carried out as in A except lane 2 shows a reaction to which cholesterol sulfate (60 μM) was readded for 20 min at room temperature after Nonidet P-40 treatment. (C) Immunoblot of NPC2 binding to FLAG–NPC1 domain 2 or FLAG–RNase H immobilized on anti-FLAG antibody resin. Myc-tagged NPC2 (2 μM), prebound with cholesterol sulfate (5 μM), was added for 30 min at 30 °C; columns were eluted with FLAG peptide (0.1 mg/mL). Eluted NPC2 protein was detected with anti-myc antibody. Mobility of molecular weight markers is indicated at left in kilodaltons. (D–G) Surface plasmon resonance of NPC1 domain 2 binding to immobilized bovine NPC2 protein at pH 5.5 (buffer B), either in the presence of 4 μM cholesterol sulfate during association and dissociation phases (D and E) or after incubation with 0.1% Nonidet P-40 followed by washing to remove detergent and without cholesterol sulfate addition (F and G). In addition, NPC2 was preincubated for 20 min at 25 °C with cholesterol sulfate (1.5-fold molar excess) prior to coupling to the chip. D–G compare binding detected at different concentrations of NPC1 domain 2.