Abstract
Background
Despite increasing therapies for moderate-to-severe psoriasis, dermatologists’ treatment preferences are unknown.
Objective
We sought to assess dermatologists’ preferences for first-line treatments and their selection determinants.
Methods
We surveyed 1000 U.S. dermatologists (500 National Psoriasis Foundation and 500 American Academy of Dermatology members who treat psoriasis) about their preferences for first-line treatment of moderate-to-severe psoriasis in healthy adults of childbearing age using standardized patient vignettes.
Results
The response rate was 39% (N=387). Preferred therapies for male and female patients were: UVB (40% and 56%, respectively), etanercept (15%, 19%), methotrexate (16%, 4%), and adalimumab (12%, 10%). Sixty-six percent of respondents administered phototherapy in their practice. After adjusting for all physician characteristics, those preferring first-line UVB for males or females were significantly more likely to have phototherapy in their practice (odds ratio (OR) 3.4, 95% confidence interval (CI) 1.8–6.6 and OR 2.8, 95% CI 1.5–5.3, respectively) and to have used UVB in more than 10 patients in the last 3 months (OR 8.0, 95% CI 3.9–16.4; OR 9.6, 95% CI 4.3–21.6). Dermatologists in the Midwest were more likely than those in the Northeast to prefer adalimumab first-line for males and females.
Limitations
We surveyed only dermatologists with interest in treating psoriasis and elicited their treatment preferences for a single base case scenario. Treatment preferences may differ between survey respondents and non-respondents.
Conclusion
UVB is most commonly preferred as a first-line treatment for moderate-to-severe psoriasis in healthy adults, and preferences vary based on region, phototherapy availability, and prior treatment use.
Keywords: psoriasis, treatment preference, first-line treatment, phototherapy, UVB, tumor necrosis factor inhibitor, methotrexate, comparative effectiveness, survey
INTRODUCTION
Psoriasis is a chronic, inflammatory disease of the skin and joints affecting 2–4% of the general population.1, 2 An estimated 1.2 million psoriasis patients in the U.S. have moderate-to-severe disease, and up to 3 million adult Americans have psoriasis but remain undiagnosed by a physician.2 Psoriasis, especially if more severe, may be a risk factor for systemic disorders including diabetes, myocardial infarction, stroke, and premature death.3–6
The treatment options for moderate-to-severe psoriasis have expanded dramatically in the last decade.7–11 Despite the growing repertoire of treatments, insufficient data exist to determine which therapies are first, second, and third line. Numerous psoriasis treatment guidelines now exist and they variably differentiate (or do not differentiate at all) between first- and second-line treatment options based on cost, risk-benefit considerations, and expert opinion.12–18 Moreover, little is known about dermatologists’ preferences for treating this disease. Such information is critical to further investigating the determinants of treatment selection and informing future comparative effectiveness studies, which have been identified as a priority by the U.S. Institute of Medicine.19
The purpose of this study was therefore to describe U.S. dermatologists’ preferences for first-line treatment in healthy adults with moderate-to-severe psoriasis using patient vignettes, a well-accepted method for measuring variation and quality in clinical practice.20–22
METHODS
Study population and setting
We conducted a survey of 1000 practicing dermatologists across the U.S.; five hundred were members of the National Psoriasis Foundation (NPF) randomly selected from the NPF’s list of 922 dermatologists and the other 500 were American Academy of Dermatology (AAD) members randomly selected from the AAD’s list of 1417 dermatologists who had identified themselves as treating psoriasis.
Study design
We conducted a survey of U.S. dermatologists as described above. The survey instrument (see online appendix) was developed by dermatologists expert in the care of psoriasis with input from steering committee members of the Dermatology Clinical Effectiveness Research Network (DCERN). First-line treatment preferences were assessed using two vignettes describing a “typical” healthy adult male or female of childbearing age with moderate-to-severe psoriasis adapted from previously published vignettes.16 For each hypothetical patient, dermatologists were asked to select their first, second, and third choices for treatment from a list of 10 biologic, oral systemic, or phototherapies currently Food and Drug Administration (FDA)-approved for the treatment of psoriasis (see appendix). The order in which treatment choices were listed was randomized in six different ways to reduce bias.
We conducted the survey using a modified Dillman Tailored Design method23, 24 of sending postcard reminders and duplicate surveys to non-respondents and randomized survey packets to include one of three financial incentives.25 The survey study was conducted from May 2010 to August 2010; all responses received within 15 weeks after the initial questionnaire mailing were included in the results.
Informed consent was obtained using the cover letter enclosed with the questionnaire. The study was approved by the University of Pennsylvania Institutional Review Board (protocol no. 810417; August 10, 2010) and reported in accordance with the STROBE statement.26
Outcomes and covariates of interest
The primary outcome of interest was dermatologists’ preferences for first-line treatment in patients with moderate-to-severe psoriasis, as indicated by the first-choice therapies selected in response to two vignettes. Information on sex and years in practice were obtained for all dermatologists surveyed using Vitals (http://www.vitals.com/, accessed 26 July 2010). We used the subjects’ mailing addresses to determine their geographical region of practice as defined by the U.S. Census Bureau (http://www.census.gov/popest/geographic/, accessed 21 November 2010). Additional respondent characteristics of interest were assessed directly via the questionnaire.
Study size
With a sample size of 1000, if 60% of respondents labeled an element as a key preference, then the width of the 95% confidence interval about that estimate would be 0.10, assuming a response rate of 40%.
Statistical analysis
Data were first summarized descriptively. Analyses of treatment preference were performed separately for the hypothetical male and female patients. Only the top four first-choice therapies were directly compared. We used Fisher’s exact tests for categorical variables and one-way analysis of variance (ANOVA) or nonparametric Kruskal-Wallis tests for continuous variables. We also performed a series of logistic regressions to evaluate interactions among covariates determined a priori to be possible predictors of treatment preference. After including all a priori variables in the initial model, we used backward elimination to remove non-significant covariates one at a time if they did not alter the other main effects by more than 10% when excluded. The final models were assessed using the Hosmer- Lemeshow goodness-of-fit test, and data points with excessive residuals were excluded in order to improve goodness-of-fit. We used two-sided tests of statistical significance (α=0.05) for all analyses. Statistical analyses were conducted using Stata/IC10 (College Station, TX).
RESULTS
Of the 1000 physicians surveyed, six were unreachable and five were considered ineligible for study inclusion because they were non-dermatologists or not currently seeing patients. Of the remaining 989 dermatologists, 655 were males and 496 were NPF members. Three hundred eighty-seven dermatologists returned the questionnaire, yielding a 39.1% response rate.
Data on sex, NPF or AAD membership status, number of years in practice, and region of practice were available for the sample population. After adjusting for all measured characteristics, survey respondents were similar to non-respondents with respect to sex, duration of practice, and geographic region. NPF members were more likely to respond than AAD members (odds ratio (OR) 2.37, 95% confidence interval (CI) 1.81–3.11). Response rates differed among the three incentive groups (results reported elsewhere),25 but we observed no meaningful variations in the respondents’ treatment preferences by incentive amount.
Physician characteristics
Survey respondents were mostly male (72%), NPF members (64%), and in private practice (70%) and represented all regions of the U.S. (Table I). Respondents had been in practice for a mean of 23.1 (standard deviation (SD) 10.6) years and had treated a median of 30 (interquartile range (IQR) 15–60) patients with moderate-to-severe psoriasis in the preceding 3 months. Sixty-six percent of dermatologists administered phototherapy in their practice. UVB, etanercept, methotrexate, and adalimumab were the treatments most heavily prescribed by responding dermatologists for their psoriasis patients (Table I). Safety and efficacy were considered “extremely” or “very” important by over 95% of respondents.
TABLE I.
Baseline characteristics of survey respondents (N=387)
| Characteristic | n (%) |
|---|---|
| Sex | |
| Female | 110 (28.42) |
| Male | 277 (71.58) |
| NPF member | |
| Yes | 246 (63.57) |
| No | 141 (36.43) |
| Region of practice in the United States | |
| Northeast | 90 (23.26) |
| Midwest | 90 (23.26) |
| South | 135 (34.88) |
| West | 72 (18.60) |
| Years in practice | |
| Overall mean (SD) | 23.1 (10.6) |
| 0–9 | 48 (12.40) |
| 10–19 | 92 (23.77) |
| 20–29 | 119 (30.75) |
| ≥ 30 | 113 (29.20) |
| Missing | 15 (3.88) |
| Practice type | |
| Academic | 40 (10.34) |
| Multi-specialty group practice | 40 (10.34) |
| Private dermatology practice (size below): | 272 (70.28) |
| Solo practice | 133 (48.90) |
| < 5 dermatologists | 97 (35.66) |
| ≥ 5 dermatologists | 37 (13.60) |
| Missing | 5 (1.84) |
| Veterans Administration | 1 (0.26) |
| Staff model HMO (i.e. Kaiser) | 2 (0.52) |
| Other | 8 (2.07) |
| Missing | 24 (6.20) |
| Physician extender (i.e. nurse practitioner, physician assistant) employed | |
| Yes | 150 (38.76) |
| Manages patients on orals or biologics? | |
| Yes | 106 (70.67) |
| No | 35 (23.33) |
| Missing | 9 (6.00) |
| No | 229 (59.17) |
| Missing | 8 (2.07) |
| Phototherapy administered by practice | |
| Yes | 255 (65.89) |
| No | 123 (31.78) |
| Missing | 9 (2.33) |
| Infusion center affiliated with practice | |
| Yes | 83 (21.45) |
| No | 295 (76.23) |
| Missing | 9 (2.33) |
| Number of moderate-to-severe psoriasis patients treated in last 3 months | |
| Median (IQR) | 30 (15–60) |
| 1st quartile (0–15 patients) | 105 (27.13) |
| 2nd quartile (16–30) | 105 (27.13) |
| 3rd quartile (31–60) | 78 (20.16) |
| 4th quartile (60–999) | 89 (23.00) |
| Missing | 10 (2.58) |
| Number of patients treated with UVB in last 3 months1 | |
| ≤ 10 patients | 267 (68.99) |
| > 10 patients | 105 (27.13) |
| Missing | 15 (3.88) |
| Number of patients treated with etanercept in last 3 months1 | |
| ≤ 10 patients | 280 (72.35) |
| > 10 patients | 90 (23.26) |
| Missing | 17 (4.39) |
| Number of patients treated with adalimumab in last 3 months1 | |
| ≤ 10 patients | 312 (80.62) |
| > 10 patients | 62 (16.02) |
| Missing | 13 (3.36) |
| Number of patients treated with methotrexate in last 3 months1 | |
| ≤ 10 patients | 302 (78.04) |
| > 10 patients | 67 (17.31) |
| Missing | 18 (4.65) |
| Importance of treatment factors (1=not at all, 5=extremely) | |
| Safety | |
| Median (IQR) rating | 5 (4–5) |
| Extremely important | 254 (65.63) |
| Very important | 119 (30.75) |
| Moderately important | 8 (2.07) |
| Somewhat important | 1 (0.26) |
| Not at all important | 0 (0) |
| Missing | 5 (1.29) |
| Efficacy | |
| Median (IQR) rating | 5 (4–5) |
| Extremely important | 224 (57.88) |
| Very important | 155 (40.05) |
| Moderately important | 3 (0.78) |
| Somewhat important | 0 (0) |
| Not at all important | 0 (0) |
| Missing | 5 (1.29) |
| Cost to patient | |
| Median (IQR) rating | 4 (3–4) |
| Extremely important | 77 (19.90) |
| Very important | 194 (50.13) |
| Moderately important | 86 (22.22) |
| Somewhat important | 21 (5.43) |
| Not at all important | 1 (0.26) |
| Missing | 8 (2.07) |
| Personal experience | |
| Median (IQR) rating | 4 (3–4) |
| Extremely important | 40 (10.34) |
| Very important | 171 (44.19) |
| Moderately important | 136 (35.14) |
| Somewhat important | 27 (6.98) |
| Not at all important | 2 (0.52) |
| Missing | 11 (2.84) |
| Ease of insurance approval | |
| Median (IQR) rating | 4 (3–4) |
| Extremely important | 66 (17.05) |
| Very important | 142 (36.69) |
| Moderately important | 120 (31.01) |
| Somewhat important | 46 (11.89) |
| Not at all important | 7 (1.81) |
| Missing | 6 (1.55) |
| Ease of administration | |
| Median (IQR) rating | 3 (3–4) |
| Extremely important | 38 (9.82) |
| Very important | 144 (37.21) |
| Moderately important | 141 (36.43) |
| Somewhat important | 50 (12.92) |
| Not at all important | 6 (1.55) |
| Missing | 8 (2.07) |
Use in >10 patients in last 3 months is defined as “heavy” use.
First-line treatment preferences
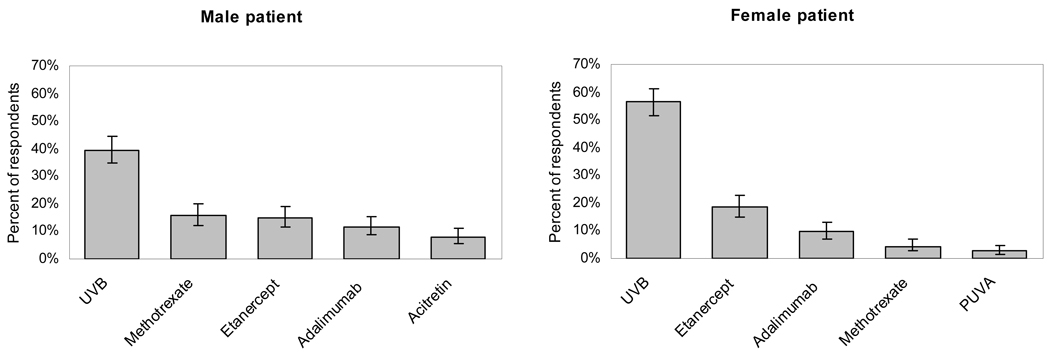
The most preferred treatments for moderate-to-severe psoriasis for the hypothetical healthy male and female patient of child-bearing potential were: UVB (39.5% and 56.3%, respectively), etanercept (15.0%, 18.6%), methotrexate (15.8%, 4.4%), and adalimumab (11.6%, 9.6%) (Figure 1). Thirty-one (8%) respondents chose acitretin as first-line treatment for males, and one respondent selected it for females. Few dermatologists preferred ustekinumab (3.1% and 1.3% for males and females, respectively), PUVA (2.1%, 2.6%), cyclosporine (0%, 1.3%), alefacept (0.3%, 0.8%) or infliximab (0%, 0.3%) as first-line therapy.
FIGURE 1. First-line treatment preferences for healthy adult with moderate-to-severe psoriasis.
Error bars indicate 95% confidence interval.
Variations in treatment preference by physician characteristics – univariate analyses
We compared the top four first-line treatment preferences by several physician factors (Table II). Compared to dermatologists in the South, those in the Northeast were significantly more likely to prefer UVB as first-line treatment for males and females (OR 2.19, 95% CI 1.19–4.05 and OR 2.13, 95% CI 1.12–4.03, respectively). Nearly 56% of dermatologists with phototherapy units in their practice selected UVB for first-line use in males, in contrast to only 29% of dermatologists without phototherapy in their practice. We observed similar differences for female patients. Compared to less frequent prescribers, dermatologists who were heavy UVB prescribers were also more likely to select UVB as their first-line therapy for both males and females. Furthermore, less frequent prescribers of etanercept, adalimumab, and methotrexate were significantly more likely to prefer UVB as first-line compared to heavy users of etanercept, adalimumab, and methotrexate, respectively.
TABLE II.
First-line treatment preferences by physician characteristic
| Male patient | Female patient | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Respondent Characteristic | UVB (N=153) n (%) |
Etanercept (N=58) n (%) |
Adalimumab (N=45) n (%) |
Methotrexate (N=61) n (%) |
p1 | UVB (N=218) n (%) |
Etanercept (N=72) n (%) |
Adalimumab (N=37) n (%) |
Methotrexate (N=17) n (%) |
p1 |
| Sex | ||||||||||
| Male | 118 (50.86) | 37 (15.95) | 28 (12.07) | 49 (21.12) | 0.045 | 162 (65.06) | 45 (18.07) | 26 (10.44) | 16 (6.43) | 0.04 |
| Female | 35 (41.18) | 21 (24.71) | 17 (20.00) | 12 (14.12) | 56 (58.95) | 27 (28.42) | 11 (11.58) | 1 (1.05) | ||
| NPF member | ||||||||||
| No | 52 (45.22) | 29 (25.22) | 17 (14.78) | 17 (14.78) | 0.08 | 70 (57.38) | 31 (25.41) | 14 (11.48) | 7 (5.74) | 0.34 |
| Yes | 101 (50.00) | 29 (14.36) | 28 (13.86) | 44 (21.78) | 148 (66.67) | 41 (18.47) | 23 (10.36) | 10 (4.50) | ||
| Region of practice | ||||||||||
| Northeast | 45 (60.81) | 18 (24.32) | 1 (1.35) | 10 (13.51) | 0.001 | 61 (76.25) | 15 (18.75) | 3 (3.75) | 1 (1.25) | 0.01 |
| Midwest | 35 (46.05) | 14 (18.42) | 15 (19.74) | 12 (15.79) | 46 (56.10) | 15 (18.29) | 15 (18.29) | 6 (7.32) | ||
| South | 46 (41.44) | 18 (16.22) | 24 (21.62) | 23 (20.72) | 74 (60.16) | 32 (26.02) | 14 (11.38) | 3 (2.44) | ||
| West | 27 (48.21) | 8 (14.29) | 5 (8.93) | 16 (28.57) | 37 (62.71) | 10 (16.95) | 5 (8.47) | 7 (11.86) | ||
| Years in practice | ||||||||||
| Mean (SD) | 23.5 (11.3) | 20.3 (8.8) | 18.9 (9.7) | 25.4 (11.4) | 0.012 | 23.8 (10.8) | 20.7 (10.2) | 20.8 (10.2) | 21.6 (9.7) | 0.102 |
| Practice type | ||||||||||
| Academic | 20 (54.05) | 4 (10.81) | 2 (5.41) | 11 (29.73) | 0.03 | 26 (70.27) | 4 (10.81) | 3 (8.11) | 4 (10.81) | 0.20 |
| Private | 105 (48.17) | 43 (19.72) | 37 (16.97) | 33 (15.14) | 151 (63.18) | 51 (21.34) | 28 (11.72) | 9 (3.77) | ||
| Multi-specialty, VA, HMO, other | 18 (40.91) | 7 (15.91) | 4 (9.09) | 15 (34.09) | 26 (55.32) | 14 (29.79) | 4 (8.51) | 3 (6.38) | ||
| Practice size (private practice only) | ||||||||||
| Solo | 44 (42.31) | 21 (20.19) | 18 (17.31) | 21 (20.19) | 0.56 | 64 (57.14) | 25 (22.32) | 16 (14.29) | 7 (6.25) | 0.36 |
| <5 dermatologists | 40 (49.38) | 17 (20.99) | 15 (18.52) | 9 (11.11) | 58 (65.17) | 20 (22.47) | 10 (11.24) | 1 (1.12) | ||
| ≥5 dermatologists | 18 (60.00) | 5 (16.67) | 4 (13.33) | 3 (10.00) | 26 (74.29) | 6 (17.14) | 2 (5.71) | 1 (2.86) | ||
| Physician extender | ||||||||||
| No | 91 (50.00) | 32 (17.58) | 21 (11.54) | 38 (20.88) | 0.49 | 133 (67.17) | 42 (21.21) | 13 (6.57) | 10 (5.05) | 0.04 |
| Yes | 59 (45.74) | 25 (19.38) | 22 (17.05) | 23 (17.83) | 81 (57.86) | 30 (21.43) | 23 (16.43) | 6 (4.29) | ||
| no pso mgmt | 15 (50.00) | 6 (20.00) | 5 (16.67) | 4 (13.33) | 0.90 | 24 (72.73) | 7 (21.21) | 2 (6.06) | 0 (0) | 0.09 |
| +pso mgmt | 39 (42.86) | 17 (18.68) | 17 (18.68) | 18 (19.78) | 52 (52.53) | 21 (21.21) | 20 (20.20) | 6 (6.06) | ||
| Phototherapy | ||||||||||
| No | 26 (28.89) | 25 (27.78) | 14 (15.56) | 25 (27.78) | <0.001 | 49 (48.04) | 32 (31.37) | 16 (15.69) | 5 (4.90) | 0.002 |
| Yes | 123 (55.66) | 32 (14.48) | 30 (13.57) | 36 (16.29) | 164 (69.49) | 40 (16.95) | 21 (8.90) | 11 (4.66) | ||
| Infusion | ||||||||||
| No | 114 (48.93) | 46 (19.74) | 32 (13.73) | 41 (17.60) | 0.36 | 168 (65.12) | 54 (20.93) | 26 (10.08) | 10 (3.88) | 0.34 |
| Yes | 34 (44.74) | 11 (14.47) | 11 (14.47) | 20 (26.32) | 44 (56.41) | 18 (23.08) | 10 (12.82) | 6 (7.69) | ||
| Number of psoriasis patients treated in last 3 mon | ||||||||||
| Median (IQR) | 30 (15–50) | 30 (15–71) | 42.5 (20–100) | 33 (18–80) | 0.213 | 30 (10–50) | 30 (20–90) | 40 (20–100) | 40 (20–75) | 0.033 |
| Heavy user of UVB4 | ||||||||||
| No | 79 (37.80) | 47 (22.49) | 38 (18.18) | 45 (21.53) | <0.001 | 128 (54.94) | 59 (25.32) | 33 (14.16) | 13 (5.58) | <0.001 |
| Yes | 70 (72.92) | 8 (8.33) | 6 (6.25) | 12 (12.50) | 83 (83.84) | 9 (9.09) | 4 (4.04) | 3 (3.03) | ||
| Heavy user of etanercept4 | ||||||||||
| No | 117 (52.47) | 33 (14.80) | 32 (14.35) | 41 (18.39) | 0.02 | 169 (68.42) | 40 (16.19) | 28 (11.34) | 10 (4.05) | 0.001 |
| Yes | 29 (35.80) | 23 (28.40) | 12 (14.81) | 17 (20.99) | 40 (47.62) | 30 (35.71) | 9 (10.71) | 5 (5.95) | ||
| Heavy user of adalimumab4 | ||||||||||
| No | 131 (51.37) | 50 (19.61) | 29 (11.37) | 45 (17.65) | 0.003 | 184 (65.95) | 59 (21.15) | 27 (9.68) | 9 (3.23) | 0.01 |
| Yes | 18 (33.96) | 6 (11.32) | 15 (28.30) | 14 (26.42) | 28 (50.00) | 11 (19.64) | 10 (17.86) | 7 (12.50) | ||
| Heavy user of methotrexate4 | ||||||||||
| No | 130 (53.50) | 46 (18.93) | 34 (13.99) | 33 (13.58) | <0.001 | 184 (67.65) | 57 (20.96) | 25 (9.19) | 6 (2.21) | <0.001 |
| Yes | 17 (28.33) | 7 (11.67) | 10(16.67) | 26 (43.33) | 24 (41.38) | 12 (20.69) | 12 (20.69) | 10 (17.24) | ||
| Importance of treatment factors5, median (IQR) | ||||||||||
| Safety | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0.733 | 5 (4–5) | 5 (4–5) | 5 (4–5) | 4 (4–5) | 0.413 |
| Efficacy | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0.623 | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0.623 |
| Cost to patient | 4 (3–4) | 4 (3–5) | 4 (4–4) | 4 (4–5) | 0.223 | 4 (3–4) | 4 (3–4) | 4 (3–4) | 4 (4–5) | 0.213 |
| Personal experience | 4 (3–4) | 4 (3–4) | 4 (3–4) | 4 (3–4) | 0.613 | 4 (3–4) | 4 (3–4) | 4 (3–4) | 4 (3–4) | 0.923 |
| Ease of insurance approval | 3 (3–4) | 4 (3–4) | 4 (3–4) | 4 (3–4) | 0.123 | 4 (3–4) | 4 (3–4) | 4 (3–4) | 4 (3–4) | 0.313 |
| Ease of administration | 3 (3–4) | 4 (3–4) | 3 (2–4) | 3 (3–4) | 0.023 | 4 (3–4) | 3 (3–4) | 3 (3–4) | 3 (3–4) | 0.593 |
p<0.05;
p<0.01;
p<0.001
Fisher’s exact test
ANOVA test
Kruskal-Wallis test
Use in >10 patients in last 3 months
1-not at all important, 5-extremely important
With respect to etanercept (Table II), factors associated with a significantly greater likelihood of preferring etanercept as first-line treatment included absence of phototherapy in the practice, heavy etanercept use in recent months, and less frequent UVB use. Factors significantly associated with a greater likelihood of selecting adalimumab for first-line use included location in the Midwest as compared to the Northeast, heavy adalimumab use (for male patient only), and less frequent UVB use (Table II). Similarly, factors significantly associated with a greater likelihood of first-line preference for methotrexate included location in the West relative to the Northeast, heavy methotrexate use, and absence of phototherapy in practice (male patient only) (Table II).
Variations in treatment preference by physician characteristics – multivariate analyses
We performed a series of logistic regressions to generate descriptive models for UVB, etanercept, adalimumab, and methotrexate preference if at least 20 respondents selected the therapy as first-line for male or female patients (Table III). The most significant physician characteristics independently associated with first-line preference for UVB were heavy use of UVB in the preceding three months and availability of phototherapy units in practices (Table III). Heavy use of etanercept or methotrexate was negatively associated with UVB preference. Male dermatologists were significantly more likely than female providers to select UVB for first-line use. As the importance of treatment cost increased, the likelihood of preferring UVB as first-line for females also increased.
TABLE III.
Series of logistic regression models predicting first-line treatment preference
| Odds Ratio (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|
| Male patient | Female patient | ||||||
| Respondent characteristic | UVB (N=324) |
Etanercept (N=315) |
Adalimumab (N=317) |
Methotrexate (N=313) |
UVB (N=321) |
Etanercept (N=317) |
Adalimumab (N=310) |
| Sex (male) | 1.94* (1.04, 3.62) |
0.39* (0.17, 0.92) |
0.31* (0.12, 0.79) |
1.51 (0.58, 3.94) |
1.94* (1.08, 3.50) |
0.44* (0.20, 0.98) |
0.30* (0.09, 0.95) |
| NPF member | 0.56 (0.31, 1.01) |
0.49 (0.22, 1.10) |
1.62 (0.69, 3.81) |
2.34 (0.99, 5.54) |
n/a | n/a | n/a |
| Region of practice (base: Northeast) | |||||||
| Midwest | 0.85 (0.27, 2.62) |
26.36** (2.88, 241.10) |
0.53 (0.15, 1.94) |
0.55 (0.24, 1.26) |
1.38 (0.47, 4.07) |
19.54** (2.86, 133.57) |
|
| South | n/a | 0.47 (0.18, 1.27) |
16.14* (1.89, 138.14) |
0.74 (0.24, 2.32) |
0.63 (0.30, 1.36) |
1.63 (0.63, 4.23) |
3.94 (0.61, 25.32) |
| West | 0.51 (0.15, 1.79) |
6.57 (0.64, 67.69) |
1.15 (0.34, 3.83) |
0.58 (0.24, 1.40) |
0.67 (0.20, 2.20) |
4.01 (0.53, 30.22) |
|
| Years in practice (base: 0–9 years) | |||||||
| 10–19 years | 0.44 (0.18, 1.06) |
0.95 (0.27, 3.28) |
1.19 (0.37, 3.88) |
2.04 (0.57, 7.26) |
0.96 (0.32, 2.85) |
0.86 (0.20, 3.72) |
|
| 20–29 years | 0.71 (0.31, 1.64) |
1.18 (0.36, 3.88) |
1.33 (0.40, 4.37) |
0.35 (0.08, 1.50) |
n/a | 1.02 (0.35, 2.96) |
1.37 (0.31, 6.13) |
| ≥30 years | 0.70 (0.29, 1.65) |
0.41 (0.10, 1.73) |
0.61 (0.14, 2.61) |
2.26 (0.63, 8.07) |
0.81 (0.24, 2.71) |
0.71 (0.13, 3.95) |
|
| Practice type (base: academic) | |||||||
| Private practice | 0.63 (0.27, 1.49) |
0.93 (0.21, 4.15) |
7.24* (1.08, 48.43) |
0.37 (0.10, 1.36) |
0.46 (0.16, 1.35) |
2.31 (0.57, 9.36) |
4.26 (0.56, 32.45) |
| Multi-specialty, VA, HMO, other | 0.44 (0.15, 1.29) |
1.37 (0.26, 7.12) |
2.03 (0.26, 16.02) |
1.57 (0.41, 5.97) |
0.25* (0.08, 0.78) |
10.17** (1.96, 52.70) |
1.27 (0.15, 10.61) |
| Physician extender hired | n/a | 0.78 (0.33, 1.83) |
1.34 (0.56, 3.21) |
n/a | n/a | n/a | 5.89** (1.95, 17.80) |
| Phototherapy in practice | 3.40*** (1.75, 6.62) |
0.65 (0.27, 1.58) |
n/a | 0.34* (0.14, 0.81) |
2.83** (1.51, 5.29) |
0.48 (0.22, 1.04) |
0.50 (0.17, 1.47) |
| Infusion center affiliation | n/a | 1.30 (0.40, 4.21) |
3.68* (1.04, 13.02) |
0.73 (0.26, 2.04) |
0.79 (0.35, 1.78) |
n/a | 4.92* (1.16, 20.81) |
| Number of psoriasis pts in last 3 | |||||||
| months (base: 1st quartile (0–15)) | |||||||
| 2nd quartile (16–30) | 1.01 (0.36, 2.82) |
2.20 (0.71, 6.84) |
0.80 (0.25, 2.51) |
0.35** (0.16, 0.75) |
2.43 (0.94, 6.27) |
3.80 (0.92, 15.66) |
|
| 3rd quartile (31–60) | n/a | 0.44 (0.13, 1.52) |
1.38 (0.38, 4.99) |
0.90 (0.27, 3.02) |
0.59 (0.26, 1.36) |
0.64 (0.20, 2.02) |
2.76 (0.61, 12.53) |
| 4th quartile (61–999) | 0.39 (0.10, 1.55) |
2.07 (0.52, 8.18) |
0.62 (0.17, 2.30) |
0.29** (0.12, 0.72) |
1.29 (0.40, 4.20) |
2.38 (0.47, 11.95) |
|
| Heavy user of UVB1 | 7.97*** (3.87, 16.42) |
0.22* (0.07, 0.71) |
0.22** (0.07, 0.68) |
0.42 (0.15, 1.20) |
9.59*** (4.25, 21.63) |
0.12*** (0.04, 0.35) |
0.14* (0.03, 0.65) |
| Heavy user of etanercept1 | 0.39* (0.17, 0.89) |
58.56*** (13.35, 256.88) |
0.52 (0.13, 2.12) |
1.50 (0.48, 4.69) |
0.26** (0.11, 0.59) |
31.75*** (9.68, 104.14) |
0.27 (0.06, 1.27) |
| Heavy user of adalimumab1 | 0.54 (0.21, 1.36) |
0.21* (0.05, 0.89) |
3.89 (0.86, 17.65) |
2.05 (0.54, 7.76) |
n/a | 0.35 (0.11, 1.17) |
n/a |
| Heavy user of methotrexate1 | 0.38* (0.16, 0.86) |
0.37 (0.10, 1.40) |
n/a | 5.78*** (2.18, 15.36) |
0.27** (0.12, 0.61) |
0.34* (0.12, 0.98) |
9.55** (2.42, 37.73) |
| Importance of safety | n/a | 0.46* (0.23, 0.91) |
n/a | n/a | n/a | 0.58 (0.31, 1.06) |
n/a |
| Importance of efficacy | n/a | n/a | 2.43* (1.03, 5.71) |
0.58 (0.29, 1.18) |
n/a | n/a | 2.56 (0.96, 6.83) |
| Importance of cost to patient | n/a | 0.70 (0.37, 1.31) |
0.99 (0.53, 1.83) |
1.61 (0.90, 2.89) |
2.12*** (1.39, 3.24) |
0.39** (0.23, 0.67) |
0.32** (0.15, 0.68) |
| Importance of personal experience | n/a | n/a | 1.04 (0.57, 1.87) |
1.05 (0.62, 1.77) |
0.86 (0.60, 1.23) |
n/a | 1.34 (0.70, 2.59) |
| Importance of insurance approval | 0.73 (0.53, 1.01) |
1.54 (0.90, 2.63) |
1.44 (0.87, 2.41) |
0.72 (0.46, 1.12) |
0.73 (0.52, 1.01) |
1.91** (1.23, 2.96) |
1.29 (0.74, 2.26) |
| Importance of ease of administration | 1.30 (0.90, 1.87) |
1.72 (0.99, 2.98) |
0.49* (0.27, 0.88) |
n/a | n/a | n/a | n/a |
p<0.05;
p<0.01;
p<0.001
Use of the particular therapy in >10 patients in last 3 months
In the case of etanercept (Table III), factors significantly associated with a greater likelihood of preferring etanercept as first-line included heavy etanercept use, less frequent UVB use, female dermatologist, lesser importance of cost (female patient only), and lesser importance of treatment safety (male patient only). Factors significantly associated with a greater likelihood of preferring first-line adalimumab included location in the Midwest as compared to the Northeast, less frequent UVB use, female dermatologist, lesser importance of cost (female patient only), and greater importance of efficacy (male patient only) (Table III). Lesser importance of treatment safety was also associated with greater (but not statistically significant) adalimumab preference (data not shown). With respect to methotrexate (Table III), the only factors significantly associated with a greater likelihood of preferring methotrexate as first-line for males were heavy methotrexate use and absence of phototherapy units in the practice.
DISCUSSION
The results of this descriptive study demonstrate several important findings. First, UVB is the most-preferred first-line treatment for both healthy males and females of childbearing potential by dermatologist respondents. The subcutaneously-administered TNF-inhibitors are the most-preferred type of biologic, and methotrexate still remains highly preferred especially for male patients. Several, but not all, guidelines specifically recommend UVB as first-line treatment (i.e. ahead of other options) for moderate-to-severe psoriasis.12–16 Nevertheless, the availability of phototherapy appears to be low relative to its preference as a first-line treatment, and phototherapy utilization is declining significantly across the U.S.27, 28
Second, treatment preference is strongly associated with factors beyond the individual patient scenario such as several physician and practice characteristics, namely recent treatment use, availability of phototherapy, and geographical region of practice. Furthermore, just as regional variations exist in the treatment of other diseases such as breast cancer and myocardial infarction,29, 30 geographical variations in first-line treatment preferences for psoriasis also exist. The driving forces behind regional variation as well as their implications for quality of care remain unknown and require future investigation.
Third, although treatment factors such as efficacy, safety, and cost were highly important to dermatologists, their effects on treatment preference were less uniform. Dermatologists who were increasingly concerned with safety were less likely to prefer etanercept first-line (similar findings were also seen for adalimumab), perhaps reflecting concerns about the potential side effects of biologics.31 Greater importance of treatment efficacy was associated with a stronger preference for adalimumab, suggesting that adalimumab may be perceived as more efficacious.32 It is possible that differences in importance of safety or efficacy were too small to be detected in our analyses as almost all survey respondents rated them extremely or very important. Interestingly, cost to the patient was a significant factor only for female patients; dermatologists were more likely to prefer UVB and less likely to prefer TNF-inhibitors as first-line if cost was more important.
As with all studies, there are limitations. First, the study design uses survey methods and is intended to be descriptive. Second, we used scripted case scenarios to elicit dermatologists’ preferences; however this is a well-accepted approach.21, 22 Third, the generalizability of our results to dermatologists who are not members of NPF or self-identified as treating psoriasis as well those who did not respond to our survey is unknown. Nevertheless, our findings are still inherently important, as they represent the stated treatment preferences of hundreds of dermatologists from across the U.S. who actively treat patients with psoriasis. Finally, we did not adjust our analyses for multiple comparisons as this was a descriptive study.33, 34
To our knowledge, this report is one of the first nation-wide studies of U.S. dermatologists’ preferences for treating moderate-to-severe psoriasis. While we describe preferences for treatment use in this study, we cannot speak to how treatments should be used. To address this latter issue, large-scale, long-term head-to-head trials directly comparing phototherapy, biologics, and traditional oral treatments are necessary.35–38 Nevertheless, we do find that despite UVB being generally preferred as first-line treatment for moderate-to-severe psoriasis in healthy adults, treatment preferences still vary based on region of practice, phototherapy availability within practices, and prior treatment experience, suggesting that there is wide variation in preference unrelated to the patient’s indication for treatment.
Acknowledgments
FUNDING SOURCES
This work was supported by grants from NIAMS RC1-AR058204 (JMG), the Doris Duke Clinical Research Fellowship (KA), and NIH Training Grant T32-AR07465 (JW).
ABBREVIATIONS
- AAD
American Academy of Dermatology
- ANOVA
analysis of variance
- CI
confidence interval
- DCERN
Dermatology Clinical Effectiveness Research Network
- FDA
Food and Drug Administration
- HIV
human immunodeficiency virus
- IQR
interquartile range
- NPF
National Psoriasis Foundation
- OR
odds ratio
- PUVA
psoralen plus ultraviolet A
- SD
standard deviation
- TNF
tumor necrosis factor
- UVB
ultraviolet B
APPENDIX. Questionnaire item assessing treatment preferences for moderate-to-severe psoriasis*
For each of the following patients, please choose the treatment you would be most likely to prescribe, assuming that all of the options are readily available and cost to the patient and insurance approval are not major issues. We understand that many factors affect prescription practices, but given the general scenario and information presented here please rank the first, second, and third treatments you would prescribe if you were required to choose.
A healthy adult male presents to you with chronic stable plaque-type psoriasis vulgaris covering >10% of his body surface area. He has not responded adequately to prior topical treatments and his psoriasis affects his quality of life.
What would you prescribe? Please rank your top three choices by filling in one circle in each column below:
| Treatment | 1st Choice (choose one) |
2nd Choice (choose one) |
3rd Choice (choose one) |
|---|---|---|---|
| Phototherapy (PUVA) | ○ | ○ | ○ |
| Phototherapy (UVB) | ○ | ○ | ○ |
| Acitretin | ○ | ○ | ○ |
| Cyclosporine | ○ | ○ | ○ |
| Methotrexate | ○ | ○ | ○ |
| Adalimumab | ○ | ○ | ○ |
| Alefacept | ○ | ○ | ○ |
| Etanercept | ○ | ○ | ○ |
| Infliximab | ○ | ○ | ○ |
| Ustekinumab | ○ | ○ | ○ |
| Other (Please specify): | ○ | ○ | ○ |
A healthy adult female of child-bearing age presents to you with chronic stable plaque-type psoriasis vulgaris covering >10% of her body surface area. She has not responded adequately to prior topical treatments and her psoriasis affects her quality of life.
What would you prescribe? Please rank your top three choices by filling in one circle in each column below:
| Treatment | 1st Choice (choose one) |
2nd Choice (choose one) |
3rd Choice (choose one) |
|---|---|---|---|
| Phototherapy (PUVA) | ○ | ○ | ○ |
| Phototherapy (UVB) | ○ | ○ | ○ |
| Acitretin | ○ | ○ | ○ |
| Cyclosporine | ○ | ○ | ○ |
| Methotrexate | ○ | ○ | ○ |
| Adalimumab | ○ | ○ | ○ |
| Alefacept | ○ | ○ | ○ |
| Etanercept | ○ | ○ | ○ |
| Infliximab | ○ | ○ | ○ |
| Ustekinumab | ○ | ○ | ○ |
| Other (Please specify): | ○ | ○ | ○ |
*Complete questionnaire is available by request from the corresponding author
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
Dr. Van Voorhees served on the advisory board of and was an investigator and speaker for Amgen and Genentech, receiving honoraria and grants; was a consultant for Incyte, Leo, VGX, and Xtrac, receiving honoraria; served on the advisory board and was a speaker for Abbott, Centocor, and Connetics, receiving honoraria; served on the advisory board and was an investigator for Bristol Myers Squibb and Warner Chilcott, receiving honoraria and grants; was an investigator for Astellas, IDEC, and Roche, receiving grants; served as a consultant for Amgen; and received honoraria from Synta. Dr. Bebo has had relationships with Abbott, Amgen, Astellas, Centocor, Galderma, Genentech, PhotoMedix, Stiefel/GSK, Warner Chilcott, and Wyeth, receiving other financial benefits. Dr. Callis Duffin was an investigator, consultant, and speaker for Abbott, Amgen, and Centocor, receiving honoraria and salary; served on the advisory board of Amgen; and received residency/fellowship program funding from Abbott and Amgen. Dr. Gelfand served as consultant and investigator with Abbott, Amgen, Centocor, Genentech, Novartis, and Pfizer, receiving grants and honoraria; was a consultant with Celgene, Covance, Galderma, Shire Pharmaceuticals, and Wyeth, receiving honoraria; and was an investigator with Shionogi, receiving grants. Drs. Abuabara, Krueger, and Troxel, Mr. Shin, and Ms. Wan have no conflicts of interest to declare.
STATEMENT ON PRIOR PRESENTATION
The data have not been presented previously.
REPRINT REQUESTS
Joel M. Gelfand, MD, MSCE, 1471 Penn Tower, One Convention Avenue, Philadelphia, PA 19104. Joel.Gelfand@uphs.upenn.edu
REFERENCES
- 1.Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136–139. doi: 10.1046/j.1087-0024.2003.09102.x. [DOI] [PubMed] [Google Scholar]
- 2.Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004. J Am Acad Dermatol. 2009;60:218–224. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 4.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 5.Gelfand JM, Troxel AB, Lewis JD, Kurd SK, Shin DB, Wang X, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493–1499. doi: 10.1001/archderm.143.12.1493. [DOI] [PubMed] [Google Scholar]
- 6.Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol. 2010;163:586–592. doi: 10.1111/j.1365-2133.2010.09941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 2001;357:1842–1847. doi: 10.1016/s0140-6736(00)04954-0. [DOI] [PubMed] [Google Scholar]
- 8.Ellis CN, Krueger GG. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med. 2001;345:248–255. doi: 10.1056/NEJM200107263450403. [DOI] [PubMed] [Google Scholar]
- 9.Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–2022. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 10.Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 11.Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, et al. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58:106–115. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–850. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 13.Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451–485. doi: 10.1016/j.jaad.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114–135. doi: 10.1016/j.jaad.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Pathirana D, Ormerod AD, Saiag P, Smith C, Spuls PI, Nast A, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23 Suppl 2:1–70. doi: 10.1111/j.1468-3083.2009.03389.x. [DOI] [PubMed] [Google Scholar]
- 16.Van Voorhees AS, Feldman SR, Koo JY, Lebwohl MG, Menter A. The psoriasis and psoriatic arthritis pocket guide: treatment algorithms and management options. Portland, OR: National Psoriasis Foundation; 2009. [Google Scholar]
- 17.Smith CH, Anstey AV, Barker JN, Burden AD, Chalmers RJ, Chandler D, et al. British Association of Dermatologists guidelines for use of biological interventions in psoriasis 2005. Br J Dermatol. 2005;153:486–497. doi: 10.1111/j.1365-2133.2005.06893.x. [DOI] [PubMed] [Google Scholar]
- 18.Nast A, Kopp IB, Augustin M, Banditt KB, Boehncke WH, Follmann M, et al. Evidence-based (S3) guidelines for the treatment of psoriasis vulgaris. J Dtsch Dermatol Ges. 2007;5 Suppl 3:1–119. doi: 10.1111/j.1610-0387.2007.06172.x. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine. Initial national priorities for comparative effectiveness research. Washington, DC: The National Academies Press; 2009. [Google Scholar]
- 20.Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA. 2000;283:1715–1722. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]
- 21.Peabody JW, Luck J, Glassman P, Jain S, Hansen J, Spell M, et al. Measuring the quality of physician practice by using clinical vignettes: a prospective validation study. Ann Intern Med. 2004;141:771–780. doi: 10.7326/0003-4819-141-10-200411160-00008. [DOI] [PubMed] [Google Scholar]
- 22.Veloski J, Tai S, Evans AS, Nash DB. Clinical vignette-based surveys: a tool for assessing physician practice variation. Am J Med Qual. 2005;20:151–157. doi: 10.1177/1062860605274520. [DOI] [PubMed] [Google Scholar]
- 23.Dillman DA. Mail and telephone surveys: the total design method. New York: Wiley; 1978. [Google Scholar]
- 24.Dillman DA. Mail and Internet surveys: the tailored design method. New York: J. Wiley; 2000. [Google Scholar]
- 25.Wan J, Abuabara K, Shin DB, Troxel AB, Bebo BF, Jr, Gelfand JM. Dermatologist response rates to a mailed questionnaire: a randomized trial of monetary incentives. doi: 10.1016/j.jaad.2011.03.017. Submitted for review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Housman TS, Rohrback JM, Fleischer AB, Jr, Feldman SR. Phototherapy utilization for psoriasis is declining in the United States. J Am Acad Dermatol. 2002;46:557–559. doi: 10.1067/mjd.2002.120451. [DOI] [PubMed] [Google Scholar]
- 28.Simpson GL, Yelverton CB, Rittenberg S, Feldman SR. Do utilization management controls for phototherapy increase the prescription of biologics? J Dermatolog Treat. 2006;17:359–361. doi: 10.1080/09546630601028786. [DOI] [PubMed] [Google Scholar]
- 29.Sariego J. Regional variation in breast cancer treatment throughout the United States. Am J Surg. 2008;196:572–574. doi: 10.1016/j.amjsurg.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Krumholz HM, Chen J, Rathore SS, Wang Y, Radford MJ. Regional variation in the treatment and outcomes of myocardial infarction: investigating New England's advantage. Am Heart J. 2003;146:242–249. doi: 10.1016/S0002-8703(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 31.Dommasch ED, Abuabara K, Shin DB, Nguyen J, Troxel AB, Gelfand JM. The risk of infection and malignancy with tumor necrosis factor antagonists in adult patients with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. Journal of the American Academy of Dermatology. doi: 10.1016/j.jaad.2010.09.734. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt J, Zhang Z, Wozel G, Meurer M, Kirch W. Efficacy and tolerability of biologic and nonbiologic systemic treatments for moderate-to-severe psoriasis: meta-analysis of randomized controlled trials. Br J Dermatol. 2008;159:513–526. doi: 10.1111/j.1365-2133.2008.08732.x. [DOI] [PubMed] [Google Scholar]
- 33.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savitz DA, Olshan AF. Describing data requires no adjustment for multiple comparisons: a reply from Savitz and Olshan. Am J Epidemiol. 1998;147:813–814. doi: 10.1093/oxfordjournals.aje.a009532. [DOI] [PubMed] [Google Scholar]
- 35.Flytstrom I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. Br J Dermatol. 2008;158:116–121. doi: 10.1111/j.1365-2133.2007.08284.x. [DOI] [PubMed] [Google Scholar]
- 36.Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118–128. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]
- 37.Heydendael VM, Spuls PI, Opmeer BC, de Borgie CA, Reitsma JB, Goldschmidt WF, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658–665. doi: 10.1056/NEJMoa021359. [DOI] [PubMed] [Google Scholar]
- 38.Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION) Br J Dermatol. 2008;158:558–566. doi: 10.1111/j.1365-2133.2007.08315.x. [DOI] [PubMed] [Google Scholar]