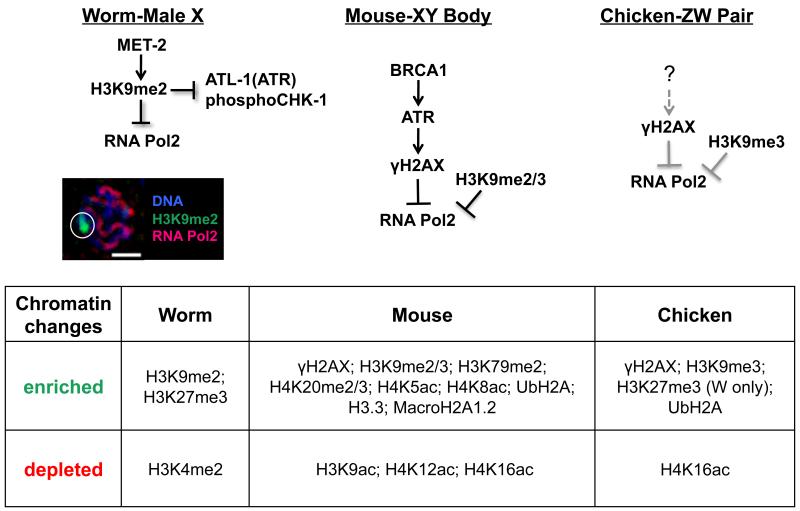
Figure 3.
Regulation of MSCI. A) Schematic of proposed molecular events targeting MSCI activation in worm, mouse, and chicken. Left: In C. elegans, the heterochromatinized male X accumulates met-2-dependent H3K9me2, a repressive mark required to block accumulation of the activated form of RNA polymerase as well as components of the recombination repair pathway, including kinases ATL-1 (ATR) and phosphorylated CHK-1 (pCHK-1). Bottom: Fluorescent micrograph of a single, C. elegans male pachytene nucleus demonstrating accumulation of H3K9me2 (green) and absence of activated RNA polymerase II (red) on the male X (identified by white circle); Scale bar=2 μM. Middle: In mouse, MSCI is activated by BRCA1-dependent ATR recruitment, which in turn phosphorylates H2AX and subsequently blocks RNA polymerase II activation, coincident with accumulation of H3K9me2/3 to the XY body (Fernandez-Capetillo et al., 2003; Turner et al., 2004). Right: In chicken, cytological analyses reveal that γH2AX and H3K9me3 are recruited to the ZW chromosomes, and RNA polymerase is excluded (Schoenmakers et al. 2009); however, whether or not this is dependent on BRCA1/ATR, as in mouse, is unknown (?). B) During MSCI, heteromorphic sex chromosomes are characterized by a number of epigenetic modifications, including the incorporation of histone variants, as well as both the acquisition and loss of sex-chromosome-specific histone modifications [see also (Zamudio et al. 2008) and (Maine 2010) for a more extensive discussion of these processes in mouse and worm].