Abstract
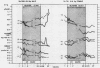
The effect of glucose infusion on rates of lipolysis were studied in a group of chair-trained papio baboons that had been prepared for chronic intravenous and intracarotid infusion. All studies were carried out after a 24 hr period of fasting and when the animals were fully awake. After a control interval of 1 hr, a glucose infusion was begun either intravenously or intra-arterially. The infusion was continued at a constant rate for 2 hr and then changed directly to the alternate route and continued an additional 2 hr. Blood samples were collected at 30-min intervals for glucose, free fatty acid (FFA), glycerol, insulin, and in some studies, growth hormone (GH) determination. When glucose doses less than 0.5 mg/kg per min were used, no change in the products of lipolysis was noted during either venous or carotid administration, and glucose and insulin levels remained stable or fell gradually. With doses of glucose between 0.5 and 0.6 mg/kg per min, a greater fall in both FFA and glycerol was noted during carotid administration. No definite changes in plasma glucose or insulin levels were noted during either infusion period. These changes in lipolysis were noted regardless of the sequence of infusion, and a similar differential suppression of FFA was noted during a 24 hr period of carotid glucose administration. When doses of glucose larger than 0.6 mg/kg per min were used, inhibition of lipolysis was noted during both phases of infusion. No definite change in GH levels was noted during the periods of fasting, and the levels of the hormone did not appear to be related to changes in glucose, insulin, or FFA levels.
These data provide additional evidence for the presence in the central nervous system of a glucose-sensitive center which alters lipolytic rates independently of insulin and GH, probably by altering sympathetic tone to adipose tissue.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CANTU R. C., WISE B. L., GOLDFIEN A., GULLIXSON K. S., FISCHER N., GANONG W. F. NEURAL PATHWAYS MEDIATING THE INCREASE IN ADRENAL MEDULLARY SECRETION PRODUCED BY HYPOGLYCEMIA. Proc Soc Exp Biol Med. 1963 Oct;114:10–13. doi: 10.3181/00379727-114-28572. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- FREDRICKSON D. S., ONO K., DAVIS L. L. LIPOLYTIC ACTIVITY OF POST-HEPARIN PLASMA IN HYPERGLYCERIDEMIA. J Lipid Res. 1963 Jan;4:24–33. [PubMed] [Google Scholar]
- FROEBERG S., LILJEDAHL S. O., OROE L. FREE FATTY ACIDS OF PLASMA DURING INSULIN-INDUCED HYPOGLYCEMIA IN DOG. THE EFFECT OF ADRENALECTOMY AND TREATMENT WITH RESERPINE, AZAMETHONIUM AND NICOTINIC ACID. Acta Med Scand. 1964 Dec;176:685–692. doi: 10.1111/j.0954-6820.1964.tb00675.x. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Ezdinli E. Z., Javid R. Effect of vagotomy and vagal stimulation on insulin secretion. Diabetes. 1967 Jul;16(7):443–448. doi: 10.2337/diab.16.7.443. [DOI] [PubMed] [Google Scholar]
- GOODNER C. J., TUSTISON W. A. AUTONOMIC MEDIATION OF THE EFFECT OF RAISED ARTERIAL GLUCOSE UPON FREE FATTY ACIDS. Science. 1964 Nov 6;146(3645):770–772. doi: 10.1126/science.146.3645.770. [DOI] [PubMed] [Google Scholar]
- GORDON R. S., Jr, CHERKES A. Unesterified fatty acid in human blood plasma. J Clin Invest. 1956 Feb;35(2):206–212. doi: 10.1172/JCI103265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodner C. J., Tustison W. A., Davidson M. B., Chu P. C., Conway M. J. Studies of substrate regulation in fasting. I. Evidence for central regulation of lipolysis by plasma glucose mediated by the sympathetic nervous system. Diabetes. 1967 Aug;16(8):576–589. doi: 10.2337/diab.16.8.576. [DOI] [PubMed] [Google Scholar]
- JEANRENAUD B., RENOLD A. E. Studies on rat adipose tissue in vitro. IV. Metabolic patterns produced in rat adipose tissue by varying insulin and glucose concentrations independently from each other. J Biol Chem. 1959 Dec;234:3082–3087. [PubMed] [Google Scholar]
- Lefebvre P. The physiological effect of glucagon on fat-mobilisation. Diabetologia. 1966 Sep;2(2):130–132. doi: 10.1007/BF00423023. [DOI] [PubMed] [Google Scholar]
- Luft R., Cerasi E., Madison L. L., von Euler U. S., Della Casa L., Roovete A. Effect of a small decrease in blood-glucose on plasma-growth-hormone and urinary excretion of catecholamines in man. Lancet. 1966 Jul 30;2(7457):254–256. doi: 10.1016/s0140-6736(66)92542-6. [DOI] [PubMed] [Google Scholar]
- McHenry L. C., Jr Cerebral blood flow. N Engl J Med. 1966 Jan 13;274(2):82–91. doi: 10.1056/NEJM196601132740206. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- ROTH J., GLICK S. M., YALOW R. S., BERSONSA Hypoglycemia: a potent stimulus to secretion of growth hormone. Science. 1963 May 31;140(3570):987–988. doi: 10.1126/science.140.3570.987. [DOI] [PubMed] [Google Scholar]
- SAKATA K., HAYANO S., SLOVITER H. A. Effect on blood glucose concentration of changes in availability of glucose to the brain. Am J Physiol. 1963 Jun;204:1127–1132. doi: 10.1152/ajplegacy.1963.204.6.1127. [DOI] [PubMed] [Google Scholar]
- SCHALCH D. S., PARKER M. L. A SENSITIVE DOUBLE ANTIBODY IMMUNOASSAY FOR HUMAN GROWTH HORMONE IN PLASMA. Nature. 1964 Sep 12;203:1141–1142. doi: 10.1038/2031141a0. [DOI] [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M., MADISON L. L. The effects of total starvation upon the levels of circulating glucagon and insulin in man. J Clin Invest. 1963 Jul;42:1031–1039. doi: 10.1172/JCI104788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIELAND O. Eine enzymatische Methode zur Bestimmung von Glycerin. Biochem Z. 1957;329(4):313–319. [PubMed] [Google Scholar]