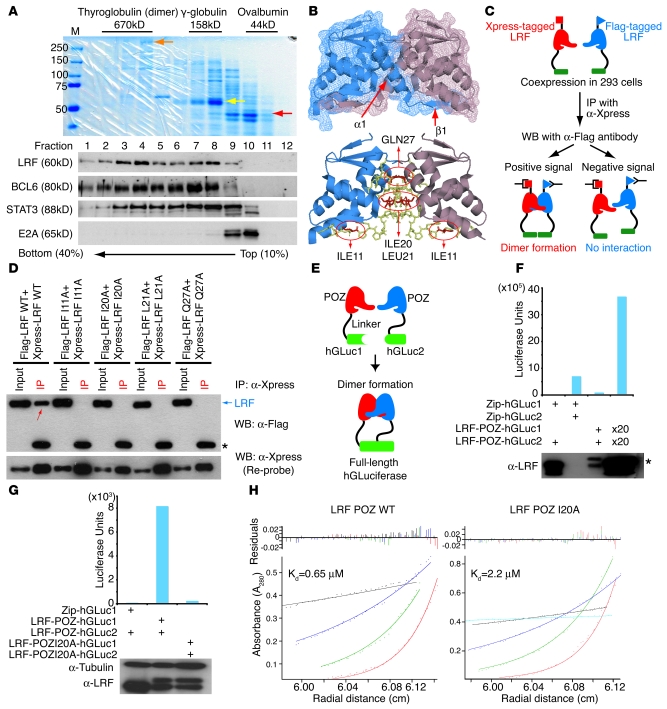
Figure 7. Identification of critical amino acid residues for LRF dimer formation.
(A) Sucrose gradient analysis of LRF in Ly-1 nuclear extracts. The mobility of molecular mass standards was visualized by staining with Coomassie blue. Red, yellow, and orange arrows represent ovalbumin (44 kDa), bovine γ-globulin heavy chain (60 kDa), and thyroglobulin (335 kDa), respectively. Western blots were also performed using the protein samples from each fraction. (B) Proposed LRF dimer structure based on the 2NN2 PDB file. 4 critical residues within the interface are indicated. ILE, isoleucine; LEU, leucine; GLN, glycine. (C) Pairs of differentially tagged WT or LRF proteins mutated in 1 critical residue were coexpressed in 293 cells and immunoprecipitated with anti-Xpress. (D) Representative Co-IP experiment. Signals indicating dimerized Flag- and Xpress-tagged LRF proteins (red arrow) are seen only when both tagged proteins are WT. Asterisk indicates IgH chain of anti-Xpress antibody. (E) Schematic representation of protein-fragment complementation assay (PCA). (F) Plasmids harboring PCA fusions were cotransfected and luciferase assays performed. Expression levels of transfected LRF-POZ proteins were determined by Western blot. 2 bands are seen in the third and fourth lanes (asterisk) because the molecular weights of LRF-POZ-hGLuc1 and LRF-POZ-hGLuc2 differ. (G) Mutant LRF-POZ protein (I20A) did not reconstitute hGLuc activity. (H) The hydrodynamic properties of the WT and I20A POZ domain were analyzed by sedimentation equilibrium experiments using AUC. Radial scans were collected at 280 nm and 20°C at 4 speeds and dimerization constants (Kd) calculated.