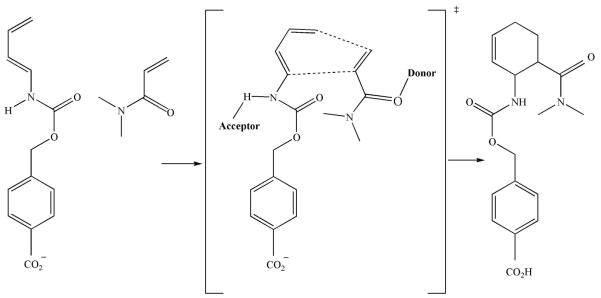
Figure 3.
A reaction scheme of the Diels-Alder reaction carried out by a designed enzyme, which catalyzes a pericyclic [4 + 2] cycloaddition of diene and dienophile to form a chiral cyclohexene ring. An acceptor and donor are required within the active site of the enzyme to activate the two molecules. The carbonyl oxygen from amino acids such as glutamine or asparagine could act as an acceptor for the diene intermediate, while a hydroxyl from amino acids such as serine, threonine or tyrosine could act as a donor for the dienophile intermediate.