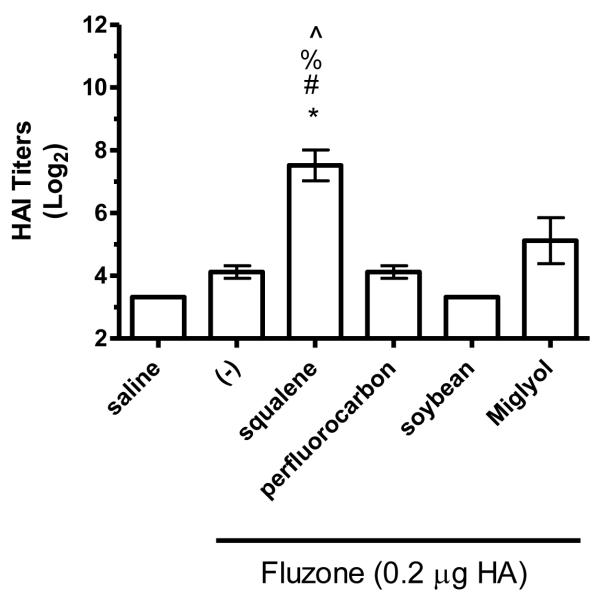
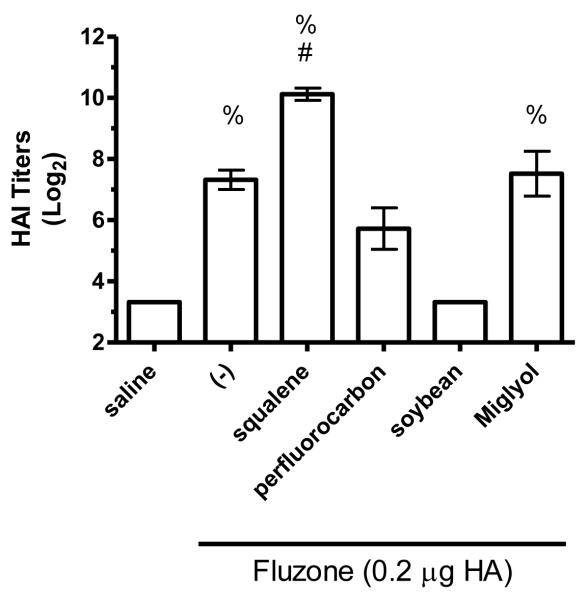
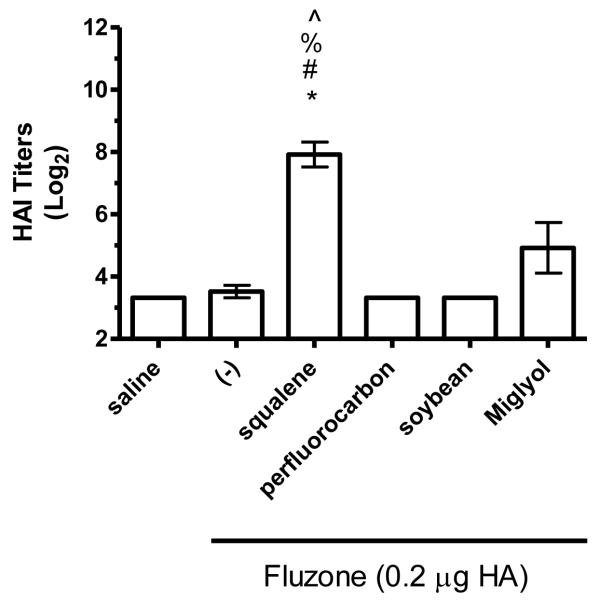
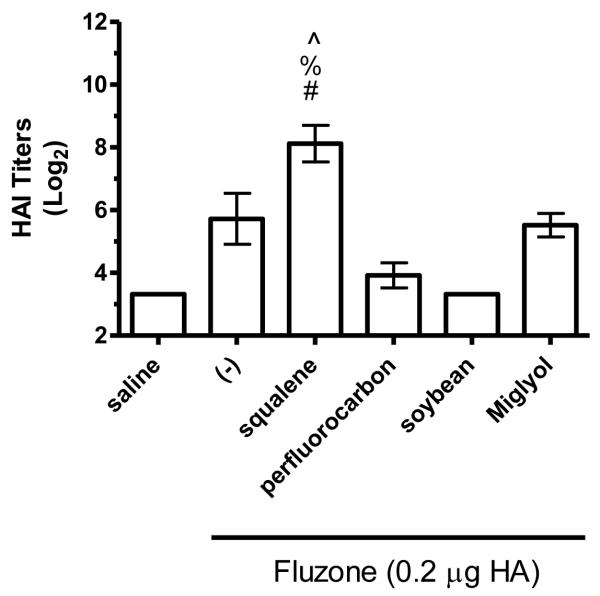
Figure 8.
HAI titers after immunization with Fluzone formulated with squalene, perfluorocarbon, soybean, or Miglyol 810 oil emulsion. BALB/c mice were immunized and then serum HAI titers determined four weeks after boosting. (a) HAI titers against the A/Solomon Islands/3/2006 (H1N1) component of Fluzone. (b) HAI titers against the A/Wisconsin/67/2005 (H3N2) component of Fluzone. (c) HAI titers against the heterologous A/Brisbane/59/07 influenza strain. (d) HAI titers against the heterologous A/Uruguay/716/07 influenza strain. Data are shown as the (Log2) titer for each individual animal, with the geometric mean and SEM represented. * = p-value < 0.05 versus immunization with vaccine alone; # = p-value < 0.05 versus immunization with the vaccine containing the perfluorocarbon emulsion; % = p-value < 0.05 versus immunization with the vaccine containing the soybean emulsion; ^ = p-value < 0.05 versus immunization with the vaccine containing the Miglyol emulsion.