Figure 6.
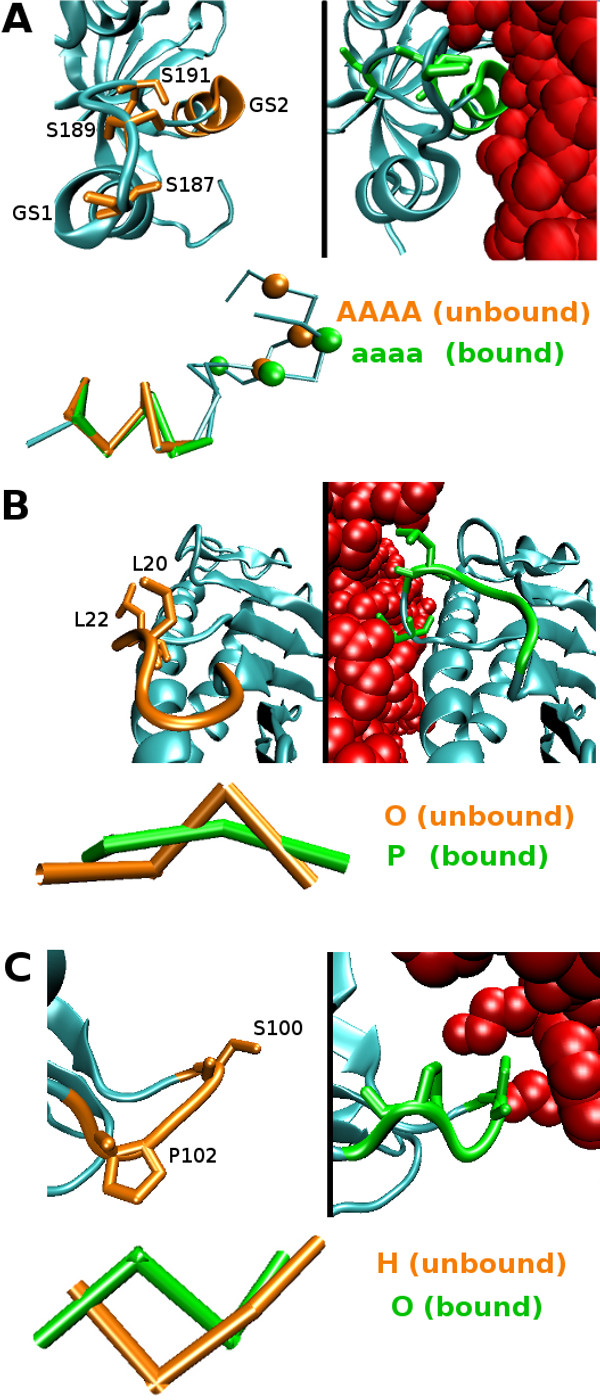
Deformation of secondary structures not fitting the deformation tendencies. A) The inhibitory conformation of the TGFβ receptor in interaction with FKBP12. The structural deformation associated with the three serines (orange balls in the unbound state, green balls in the bound state) of the phosphorylation site and to the αGS2 from a straight α-helix encoded by a run of [A] in the structural sequence (orange) to a curved α-helix encoded by a run of [a] upon interaction (green) are represented. B) Deformation of the α1 domain loop of HFE upon complexation with TfR from an unbound curved (orange) to a bound extended (green) conformation enabling a higher exposure of L20 and L22 (licorice representation) to the protein exterior and the interaction of L22 with TfR (red). C) Deformation of a surface loop on the transthyretin surface upon interaction with a retinol-binding protein (red) from an unbound straight conformation (orange) to a bound curved one (green), where S100 (licorice representation) is pushed towards the protein interior upon complexation.
