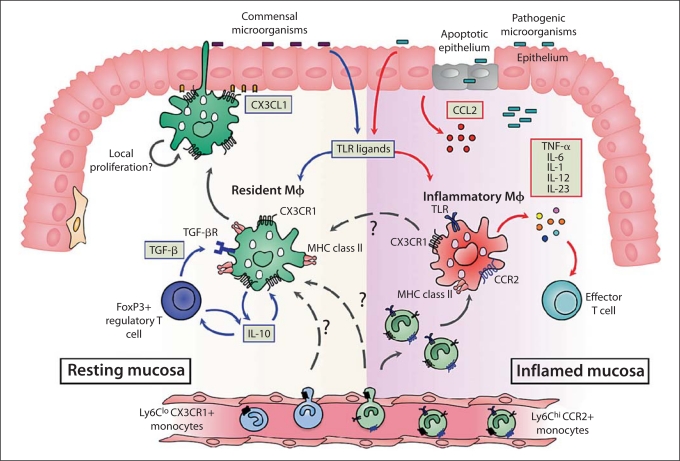
Fig. 4.
Origin and diversity of intestinal mΦ in healthy and inflamed intestine. In resting intestine, the majority of intestinal mΦ express high levels of CX3CR1, the receptor for the transmembrane chemokine fractalkine (CX3CL1) expressed by intestinal epithelial cells. These mΦ can ingest and kill commensal bacteria, perhaps by extending processes across the epithelial barrier into the lumen. They produce IL-10 constitutively, but are unable to produce pro-inflammatory mediators in response to TLR or other stimuli. This means that resident intestinal mΦ cannot assist effector T cell activity, but instead, their production of IL-10 is needed to maintain the survival of local FoxP3+ Treg. In turn, the Treg produce TGF- β which, together with IL-10 and other local factors, maintain resident mΦ in their state of partial inertia. This may also require TLR signalling from the commensal microbiota. The resident mΦ are long-lived, and may be replenished directly from the bloodstream either by resident CX3CR1+ Ly6Clo monocytes or by inflammatory CX3CR1lo Ly6Chi monocytes. There may also be local turnover of resident mΦ, or differentiation of the small numbers of CX3CR1int inflammatory mΦ present in normal mucosa (see text for details). After breach of the epithelial barrier, or pathogenic invasion, the mucosa is infiltrated by large numbers of inflammatory mΦ which express lower levels of CX3CR1. These CX3CR1int mΦ are derived from recently divided inflammatory CX3CR1lo Ly6Chi CCR2+ monocytes in the bloodstream, recruited in response to increased levels of CCR2 ligands. The CX3CR1int mΦ produce large amounts of pro-inflammatory mediators that drive local inflammation and promote the function of effector T cells. Some inflammatory CX3CR1int mΦ may eventually differentiate into resident CX3CR1hi mΦ to assist resolution of damage.