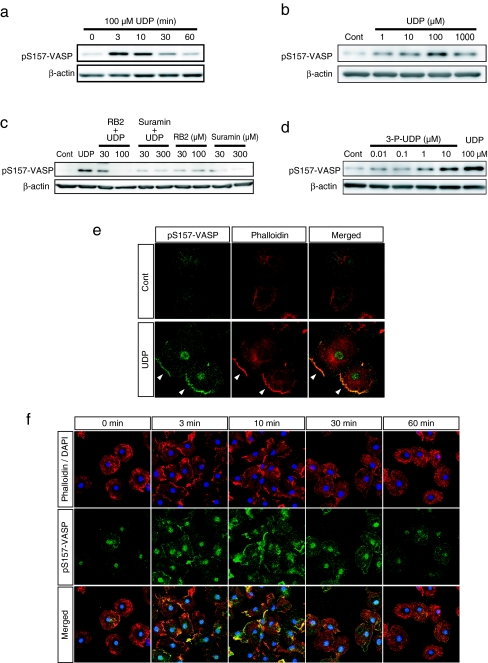
Fig. 2.
UDP induced the phosphorylation and membrane accumulation of VASP. a The cells were stimulated with UDP (100 μM) for the indicated time and then lysed. The phosphorylated VASP in the extracted cellular protein was detected by Western blot analysis using the pS157-VASP antibody. The phosphorylation of VASP at Ser157 was increased from 3 min after UDP stimulation and returned to basal level around 30 min. b The cells were stimulated with UDP at indicated concentrations for 3 min. Phosphorylated VASP was increased from 1 μM UDP and peaked at 100 μM. c The cells were pretreated with P2 receptor inhibitors, RB2 and Suramin at indicated concentrations for 10 min and then stimulated with UDP (100 μM) for 10 min. Western blot analysis revealed that UDP-induced VASP phosphorylation was suppressed by each P2 receptor inhibitor. d The cells were stimulated with P2Y6 receptor agonist 3-P-UDP at indicated concentrations or UDP at 100 μM for 3 min. 3-P-UDP also induced VASP phosphorylation from the concentration of 1 μM; at 10 μM the increased phosphorylation was comparable to UDP stimulation. e The cells were stimulated with or without UDP (100 μM) for 3 min and stained with pS157-VASP antibody (green) and phalloidin (red). UDP stimulation induced the accumulation of phosphorylated VASP to the periphery of the cell where F-actin aggregated (as indicated by arrow heads). f Time course of phosphorylated VASP localization after UDP stimulation. UDP stimulated the accumulation of phosphorylated VASP to the plasma membrane in a time-dependent manner, which peaked at 3 to 10 min after stimulation and correlated with the time course of actin aggregation