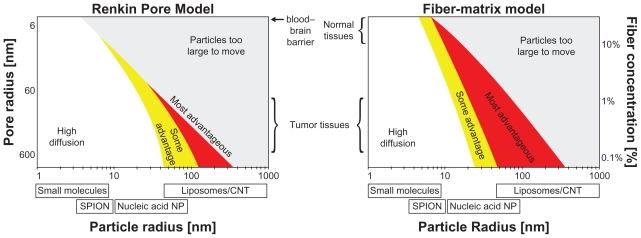
Figure 1.
A map of when dynamic magnetic shift (DMS) is predicted to be advantageous over diffusion alone for poorly perfused liver metastases (for a sample 0.5 mm diameter tumor, therapeutic particles are assumed to have a 45-minute in vivo residence time). For two common types of tissue models, a Renkin Pore model19,20,24 or a Fiber-Matrix model,19,20,25 the coloring shows when DMS treatment will improve drug delivery to the tumor. Here, “High diffusion” refers to the region where diffusion alone should suffice. It is the region where particle diffusion is predicted to create a concentration of therapy in all tumor cells that is ≥85% of the concentration of therapy in the bloodstream. “Some advantage” (yellow) and “Most advantageous” (red) is where diffusion will not suffice and DMS has the potential to improve therapy concentration to all cells in the tumor by >17% and >100%, respectively, compared with diffusion alone. Thus, DMS will be advantageous for mid-range 10–500 nm particle sizes, when the particles are big enough that diffusion alone is no longer effective but small enough that they can be magnetically moved through tissue. Particles of this size include heat shock protein cages (<16 nm),26 polymeric micelles (<50 nm),27 colloidal suspensions of albumin-Taxol (Abraxane, 130 nm),28 and functionalized carbon nanotubes (0.1–4 μm).29