Fig. 4.
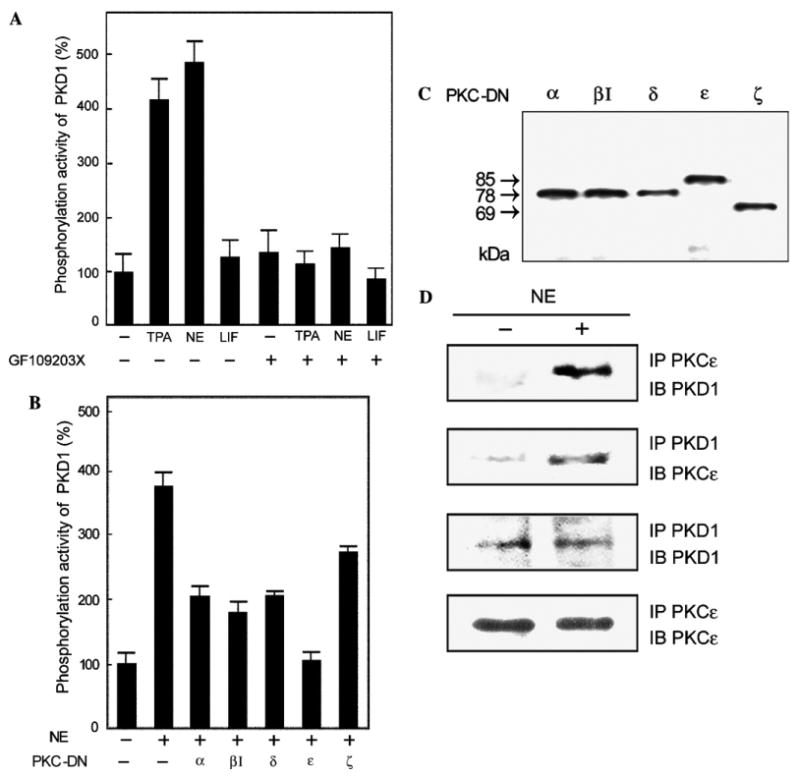
PKC-dependent activation of PKD1, effect of dominant negative PKC isoforms, and association of PKD1 and PKCε. (A) Neonatal rat cardiomyocytes were treated with either 20 nM TPA, 100 μM NE plus 2 μM propranolol, or 20 μM LIF with (+) or without (−) 400 nM GF1 for 20 min. PKD1 in the cell lysates was immunoprecipitated using an anti-PKD1/2 monoclonal antibody and its phosphorylation activity was measured as described in Materials and methods. The mean percentage of PKD1 activity compared to that of the control cells is shown with the standard deviation (n = 9). (B) The cells were transfected with plasmids for various PKC-DN isoforms (α, βI, δ, ε, and ζ) by using Duo Fect and stimulated with 100 μM NE plus 2 μM propranolol for 20 min (+). PKD1 was then immunoprecipitated and subjected to kinase assay as above (n = 9). (C) The cell lysates used in (B) were subjected to 10% SDS–PAGE and then analyzed by immunoblotting using an anti-HA monoclonal antibody (12CA5). (D) The cells were stimulated with 100 μM NE plus 2 μM propranolol for 20 min (+). Immunoprecipitates for PKCε or PKD1 were subjected to 10% SDS–PAGE, and then analyzed by immunoblotting with an anti-PKCε or anti-PKD1 antibody.
