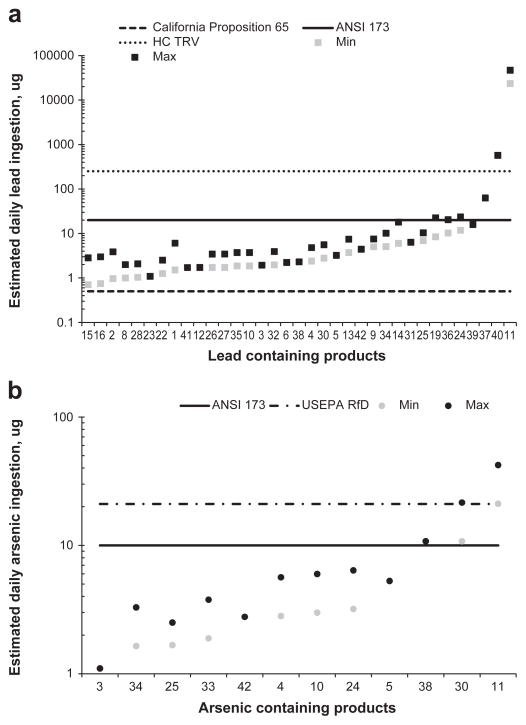
Fig. 2.
Estimated daily ingestion amounts of (a) lead and (b) arsenic for Ayurvedic medicines containing bioaccessible lead and arsenic. Min = minimum amount based on minimum dose; max = maximum amount based on maximum dose. The California Proposition 65 maximum allowable dose level for lead is 0.5 μg.d−1; the American National Standard Institute/National Sanitation Foundation International Dietary Supplement Standard 173 (ANSI 173) states that dietary supplements should not contain undeclared metals that would cause intakes greater than 20 μg.d−1 for lead and 10 μg.d−1 for arsenic; the Health Canada Toxicological Reference Value (HC-TRV) for lead is 0.0036 mg.kg−1.d−1 corresponding to 252 μg.d−1 for a 70 kg person; the United States Environmental Protection Agency reference dose (USEPA RfD) for chronic oral intake of arsenic is 0.3 μg. kg−1.d−1, corresponding to 21 μg.d−1 for a 70 kg adult.