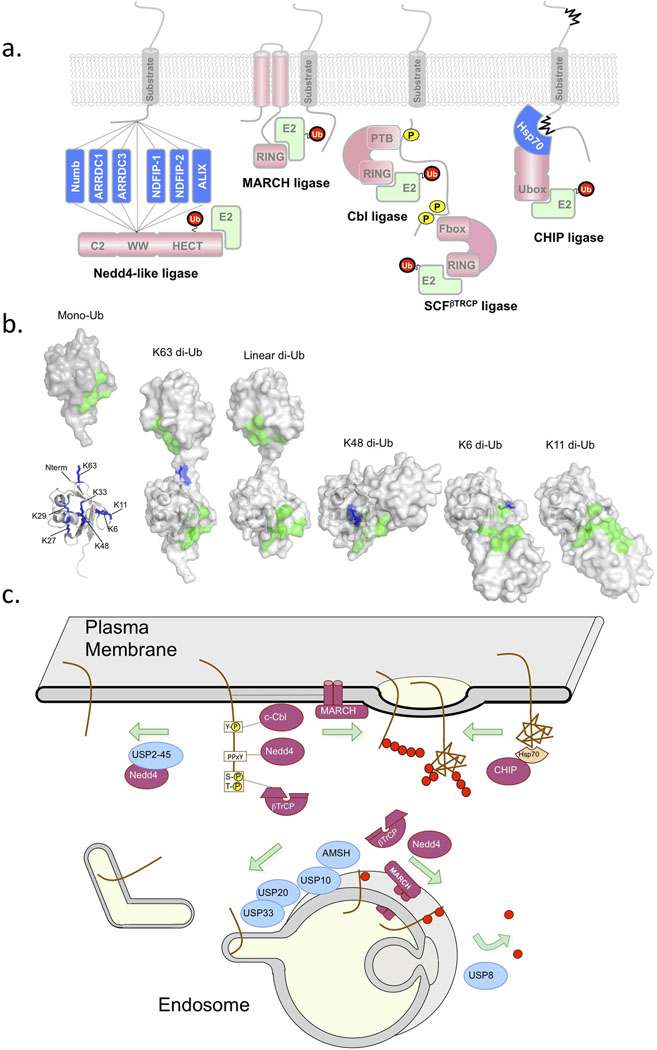
Figure 1. Ubiquitination and Deubiquitination for Lysosomal Sorting.
Several key enzymes have been found to play major roles in controlling the ubiquitination and deubiquitination of cargo. a) Shows a schematic of Ub ligases. Nedd4 family ligases bind to substrates or substrate adaptors via their WW domains [69]. Nedd4 family ligases use the HECT catalytic domain for Ub transfer, which covalently carries Ub via a thioester bond before transfer to substrates [68]. Other Ub ligases have RING domains or Ubox domains that associate with E2 conjugating enzymes and that position the E2 conjugating enzyme which bears the thioester-linked Ub near the substrate. Many RING-containing MARCH Ub-ligases have transmembrane domains, and may use these domains as means to recognize membrane protein substrates. Other RING ligases such as Cbl and Tripartate Skp1/Cullen/Fbox complexes such as SCFβTrCP can recognize phosphorylated cell surface via a phosphotyrosine binding domain (PTB) or other modules that bind phosphorylated serine/threonine residues [92, 93]. The ligase CHIP uses a Ubox to associate with an E2 conjugating enzyme. CHIP forms a complex with the chaperone Hsp70 to recognize and ubiquitinate unfolded damaged proteins fulfilling an important role in peripheral quality control [94]. b) Mono-Ub (left) has an interaction surface (green) centered around L8, I44, R42, H68, and V70 which is involved in binding the majority of Ub-binding domains [1]. Ub also has 7 lysines (blue: 6,11,27,29,33,48,63) as well as the N-terminus that can act as conjugation sites for other Ub molecules via an isopeptide bond with the C-terminus G76 residue [2]. Structures of di-Ub linked via different lysines show dramatically different conformations and position the tandem interaction surfaces (green) within each Ub moiety in different orientations that might be differentially recognized by Ub-binding domains and/or DUbs. c) shows a schematic of where key E3 Ub-ligases and counteracting DUbs are thought act in the endocytic pathway. Either exerting their control at the cell surface to determine whether a cell surface protein undergoes clathrin mediated internalization or whether it undergoes sorting into intralumenal vesicles of MVB/endosomes.