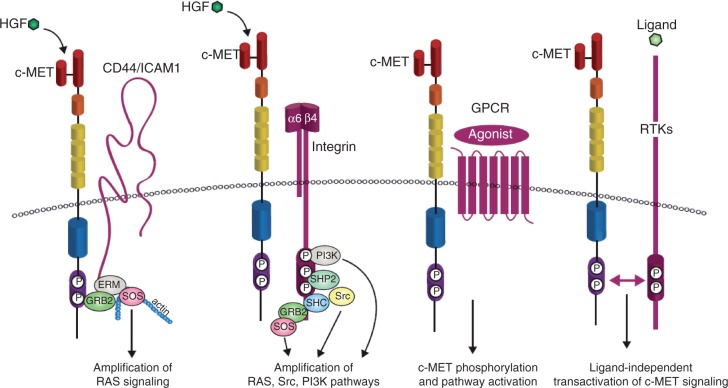
Figure 3.
c-MET transactivation. The potency and endurance of c-MET-triggered pathways is secured by a network of upstream signaling co-receptors that physically associate with c-MET at the cell surface. c-MET membrane partners can then amplify and/or diversify c-MET-dependent biochemical inputs and translate them into meaningful (and specific) biological outcomes. The v6 splice variant of the hyaluronan receptor CD44 links c-MET signaling to the actin cytoskeleton via the growth factor receptor-bound protein 2 (GRB2) and the ezrin, radixin, moesin (ERM) family of proteins in order to recruit son of sevenless (SOS), which then amplifies RAS-ERK signaling. Intercellular adhesion molecule 1 (ICAM-1) can substitute for CD44v6 as a co-receptor for c-MET in CD44v6 knockout mice, resulting in similar c-MET pathway activation. c-MET binding to integrin α6β4 creates a supplementary docking platform for the binding of signaling adaptors, leading to specific enhancement of phosphatidylinositol 3-kinase (PI3K), RAS and SRC activation. c-MET can also be activated by G-protein coupled receptors (GPCRs), although the functional outcome of this interaction is not well characterized. Crosstalk between c-MET and other receptor tyrosine kinases (RTKs) has also been studied in great depth because of its potential importance in the development of resistance to cancer therapeutics. Examples of these RTKs include the semaphorin receptors, the epidermal growth factor receptor (EGFR) family of receptors, the recepteur d'origine nantais (RON), platelet-derived growth factor receptor (PDGFR) and Axl; the list continues to grow. Adapted from Trusolino et al. [2010] and Corso et al. [2005] HGF, hepatocyte growth factor; SHC, src homolgy 2 domain-containing; SHP2, src homology 2 domain-containing phosphatase 2.