Abstract
Background
The hypothalamic pituitary adrenal (HPA) axis is a major stress response system hypothesized to be involved in the pathogenesis of posttraumatic stress disorder (PTSD). However, few studies have prospectively examined the relationships among pre-exposure HPA activity, acute stress reactions and PTSD symptoms.
Methods
Two hundred and ninety-six police recruits were assessed during academy training prior to duty-related critical incident exposure and provided salivary cortisol at first awakening and after 30 minutes. A measure of cortisol awakening response (CAR) was computed as the change in cortisol level from the first to the second collection. At 12, 24, and 36 months following the start of active police service, officers were assessed for peritraumatic distress, peritraumatic dissociation, ASD symptoms, and PTSD symptoms to their self-identified worst duty-related critical incident. Mixed models for repeated measures were used to analyze the effects of CAR on the outcome variables pooled across the three follow-up assessments.
Results
Mixed model analyses indicated that after controlling for time of awakening, first awakening cortisol levels, and cumulative critical incident stress exposure, CAR during academy training was associated with greater peritraumatic dissociation, β=.14, z=3.49, p<.0001, and greater acute stress disorder (ASD) symptoms during police service assessed at 12, 24, and 36 months, β=.09, z=2.03, p<.05, but not with peritraumatic distress β=.03, z=.81, p=.42 or PTSD symptoms β=−.004, z=−.09, p=.93.
Conclusions
These findings suggest that greater cortisol response to awakening is a pre-exposure risk factor for peritraumatic dissociation and ASD symptoms during police service.
Keywords: Cortisol, Police Stress, Peritraumatic Dissociation, Acute Stress Disorder, Posttraumatic Stress Disorder
Introduction
Traumatic stress exposure often results in peritraumatic stress responses during and in the immediate aftermath of trauma and in subsequent acute and posttraumatic stress reactions in a proportion of those exposed. Peritraumatic reactions can include emotional distress (e.g., fear, helplessness, horror, and anger), physiological reactivity (e.g., racing heart, sweating, and shaking) (1) and dissociation (e.g., emotional numbing, reduced awareness of one’s surroundings, depersonalization, derealization, amnesia) (2, 3). Acute Stress Disorder (ASD) reactions include dissociative symptoms, reexperiencing, avoidance, and anxiety and/or arousal with distress or impairment that are prolonged for a minimum of 2 days and a maximum of 4 weeks (4). Posttraumatic stress disorder (PTSD) is considered when intrusions, avoidance and numbing, and hyperarousal symptoms are prolonged for at least one month (4). ASD is a strong predictor of the subsequent development of PTSD, although many individuals with PTSD do not have histories of ASD (5).
Peritraumatic reactions are believed to be driven by excessive activation of the basolateral nucleus of the amygdala and other paralimbic structures and insufficient activation of emotion modulating centers in the prefrontal cortex, which collectively result in more severe and prolonged states of threat driven activation (6, 7). This excessive activation and resultant sympathetic activity is believed to enhance fear conditioning and memory consolidation, subsequently resulting in chronic hyperarousal symptoms that are characteristic of ASD and PTSD (8). Since the hypothalamic-pituitary-adrenal (HPA) axis is a key regulator of the stress response, with greater cortisol response serving to constrain adrenergic activation, it has been hypothesized that deficits in activation of the HPA axis (e.g., responding to the stressor with lower levels of cortisol secretion) during the peritraumatic period is involved in the pathogenesis of PTSD (9).
Cortisol secretion typically follows a circadian pattern, increasing in the second half of the night, peaking in the early morning and then declining throughout the day (10). Superimposed on this circadian pattern are secretory episodes, a major one being the cortisol awakening response (CAR), defined here as the change in cortisol levels from first awakening to 30 minutes after awakening. Recently, increased attention has been placed on the CAR as an HPA axis marker associated with psychological stress reactivity. Awakening is associated with the activation of multiple brain arousal circuits and produces a distinct rise of cortisol levels by around 50% that occurs within 20-30 minutes after awakening in approximately 75% of individuals (11, 12). The magnitude of the CAR has been found to be associated with acute and chronic stress (13, 14). Cross-sectional studies have also suggested an association between current PTSD and lower CAR (15-17), although a study of women experiencing ongoing intimate partner violence found a higher CAR associated with PTSD symptoms (18). Similarly, studies have also reported that lower CAR or morning cortisol levels were associated with peritraumatic distress or peritraumatic dissociation (17, 19).
Several studies, although not all (e.g., 20), found an inverse relationship between cortisol levels in the first hours following exposure to a traumatic stressor and the later development of PTSD symptomatology in adults (21, 22). Lower immediate cortisol levels were also associated with greater initial stress reactions (e.g., intrusive thoughts) (22). While these studies suggest that HPA axis alterations in the hours following exposure are present in those who develop persistent PTSD symptoms, it is unclear whether these alterations exist prior to the index event or are a consequence of exposure since cortisol was measured shortly after the traumatic event and probably while participants were still experiencing acute stress. Only one study to date assessed HPA axis functioning prior to trauma exposure. Heinrichs and colleagues (23) examined awakening and diurnal salivary cortisol during training in 43 firefighters and PTSD symptoms 3, 6, 9, 12, and 24 months after starting firefighting service but found no association. However, the small sample size at follow-up may have limited their ability to detect significant relationships.
The present study examined whether the magnitude of the CAR prior to trauma exposure predicts the later development of peritraumatic and posttraumatic symptoms in a study of police officers. Because PTSD has been associated with enhanced negative feedback of the HPA axis (24), we predicted that recruits with smaller increases in awakening cortisol during police academy training would have greater peritraumatic distress, dissociation, ASD symptoms, and PTSD symptoms related to their self-identified most distressing critical incident assessed at 12, 24, and 36 months following the start of active police service.
2. Methods and Materials
2.1 Participants
Data were collected as part of an ongoing prospective study of police officer stress and health of 400 police recruits during academy training in four urban police departments (New York; Oakland, San Francisco, and San Jose, CA). Complete self-report, interview, and cortisol data were obtained from 356 recruits at baseline, and 296 individuals completed at least one follow-up (completed follow-ups at 12 months: n=263; 24 months: n=220; 36 months: n=204). The ASD scale was introduced at a later start date and is only available on a subsample (n=168 to 185 at months 12, 24, and 36). There were no significant differences between participants retained to 36 months and those lost to follow-up on gender, ethnicity, income, education, or on distress at baseline as measured by the SCL-90 (25). However, younger recruits were less likely to complete a follow-up assessment, t(112.29)=2.68, p<.01.
2.2 Procedures
2.2.1 Sampling and Recruitment
Academy trainees were introduced to the study through a presentation made by study personnel during academy training classes, letters from the commissioner or police chief, and informational flyers posted at the academies. Trainees who had previously served in the military, law enforcement or emergency services were excluded. Procedures were approved by the University of California, San Francisco Institutional Review Board and a Federal Certificate of Confidentiality was obtained.
Participants were evaluated at baseline (academy training), and 12, 24, and 36 months after commencement of training. Prior to the initial assessment, study procedures were described in detail and written informed consent was obtained.
2.3 Measures
2.3.1 Initial Assessment During Academy Training
The initial assessment involved a structured clinical interview conducted by Ph.D. level clinicians and self-report questionnaires. Current or past mood disorders, anxiety disorders, and substance use disorders were determined by interview with the Structured Clinical Interview for DSM-IV, version 2.0 (SCID I-NP) (26) and familial alcohol and drug related difficulties were assessed using the Family History Screen (FHS) (27). Participants completed the self-report Symptom Checklist 90-Revised (SCL-90-R) (28) General Symptom Index (GSI) for determining levels of general psychiatric symptoms and the Pittsburgh Sleep Quality Index (PSQI), a 19-item self-report questionnaire to assess sleep quality and disturbances during the previous month (29). Lifetime Exposure to Stressful Events was assessed by interview using the Life Stressor Checklist-Revised (30). Respondents indicated 1) whether the event occurred; 2) whether the event triggered intense emotions consistent with DSM-IV criterion A2 for PTSD; 3) frequency of each event; and 4) ages of first and last occurrence. We considered participants as trauma-exposed if they experienced personal life-threat or physical harm to the self, maintaining consistency with previous research (31).
Salivary Cortisol
Saliva collection is a well-validated and unobtrusive method of measuring cortisol that reflects level of free serum cortisol (32). Subjects were instructed to collect samples at 0 and 30 minutes after awakening, to maintain a stable sleep-wake schedule during the testing, to perform this procedure in the middle of their working week, and to abstain from activities such as brushing or flossing their teeth, smoking, exercising, and ingesting food or drink prior to or during the collection. CAR was calculated as the change in log-transformed cortisol levels between 0 to 30 minutes (33). Samples were collected at home using Salivettes (Sarstedt Inc, Newton, NC), returned by mail, and deep-frozen (−78°C) until assay. We relied on participant report for information about the time of awakening and sampling.
2.3.2 Follow-Up at 12, 24, and 36 months
Participants were assessed at 12, 24 and 36 months after the start of active police duty with the following measures:
Critical Incident Exposure
The Critical Incident History Questionnaire (CIHQ) is a 34-item self-report measure assessing cumulative exposure to critical incidents in the line of duty (34). The frequency of exposure to each critical incident is tabulated, resulting in a total cumulative exposure score across all items.
Stress Symptoms
The Acute Stress Disorder Scale (ASDS) is a 19-item self-report measure assessing dissociation, intrusions, avoidance, and hyperarousal based on the DSM-IV criteria for ASD (4) in the 4 weeks following participants self-identified worst critical incident. The PTSD Checklist-specific (PCL-S) is a well-established 17-item self-report assessment of DSM-IV based PTSD-related symptoms of intrusion, avoidance, and hyperarousal that has been used in numerous studies (35, 36). Responses to the PCL-S reflected current symptom levels present during the week of the 12, 24, or 36 month assessment that were related to respondents’ worst critical incident that may have occurred at any time during police service.
Peritraumatic Reactivity
Peritraumatic reactions were assessed at 12, 24, and 36-month follow-up visits and reflected peritraumatic reactions related to officer’s self-identified worst critical incident occurring at any point during police service. Measures included the Peritraumatic Distress Inventory (PDI), a 13-item self-report questionnaire measuring dysphoric emotional states such as fear and physiological responses during and immediately after trauma exposure (1) and the Peritraumatic Dissociative Experiences Questionnaire (PDEQ), a 10-item instrument assessing immediate dissociative responses to traumatic exposure (3).
2.4 Data Analysis
All data were checked for expected ranges, presence of outliers and abnormal values, and to determine that the distribution of variables met assumptions of statistical tests. Cortisol levels were found to have substantial skew and were transformed using the logarithm (to the base 10) prior to analysis. Following transformation, two observations (one time point for each of two subjects) were dropped from the analysis because they were multivariate outliers, with mixed model residuals exceeding four standard deviations above or below the mean.
Subsequent analyses (e.g., correlational analyses or independent sample t-tests) were conducted to identify potential confounds such as age, time of first awakening, smoking, body mass index, personal or familial substance disorders, childhood trauma and cumulative severity of critical incident stress exposure (assessed separately at 12, 24 and 36 months reflecting cumulative exposure for the previous year) on baseline cortisol measures and symptom outcomes.
Linear mixed models for repeated measures were used to test the main hypotheses that a smaller increase in awakening cortisol during police academy training would be associated with greater peritraumatic distress and dissociation, and ASD and PTSD symptoms related to their most distressing critical incident assessed at each of the 3 yearly follow-up sessions following the start of active police servicei. The models included a random intercept to accommodate correlations in the outcome variables over time within each subject. All other predictors and covariates, including time point, were specified as fixed effects. Model coefficients represent the effect of each predictor pooled over all three yearly follow-up assessments.
3. Results
3.1 Sample Characteristics
Sample baseline characteristics are described in Table 1 and critical incident stress exposure and symptoms over the first three years of police service are shown in Table 2. SCID interviews confirmed low rates of current or lifetime Axis I disorders during or prior to academy training (Current disorders included: depression NOS (1), anxiety NOS (1); Lifetime disorders included: mood disorder due to a general medical condition (1), anxiety disorder due to a general medical condition (1), specific phobia (1), major depression (18), depression NOS (7), PTSD (2), alcohol abuse (29), and alcohol dependence (8)). Family history of alcohol and drug use disorders in first-degree relatives was endorsed by 15.3% (42) and 9.1% (25) of officers, respectively. Over the course of three years, 98% of officers reported exposure to one or more critical incident stressors on the CIHQ and 77% experienced one or more personally life threatening events.
Table 1.
Sample Characteristics at Baseline (N = 293-296)
| N (%) or M (SD) | |
|---|---|
| Age | 27.40 (4.97) |
| Female Gender | 42 (14.2%) |
| Education | |
| Up to 12th grade | 3 (1.0%) |
| HS diploma/GED | 31 (10.5%) |
| A.A./B.A./B.S. | 247 (84.0%) |
| M.A./ M.S./ Ph.D. | 12 (4.4%) |
| Ethnicity | |
| Caucasian | 116 (39.6%) |
| Hispanic/Latino | 67 (22.9%) |
| Asian/Pacific Islander | 44 (15.0%) |
| African-American/African | 37 (12.6%) |
| Other/Mixed | 29 (9.9%) |
| Prior Civilian Trauma Exposure | 149 (57.3%) |
| Body Mass Index | 25.72 (3.50) |
| Awakening Cortisol Level (nmol/l) | 23.89 (12.76) |
| Cortisol Awakening Response (nmol/l) | 7.05 (16.08) |
A.A., Associate in Arts; B.A., Bachelor of Arts; B.S., Bachelor of Science; GED, General Educational Development; HS, high school; M.A., Master of Arts; M.S., Master of Science; PDI, Peritraumatic Distress Inventory; Ph.D., Doctor of Philosophy; SD, standard deviation.
Table 2.
Critical Incident Stress Exposure and Symptoms Over 3 Years of Police Service (N = 293-296)
| 12 months N (%) or M (SD) |
24 months N (%) or M (SD) |
36 months N (%) or M (SD) |
|
|---|---|---|---|
| Exposed to critical incidents each year | 281 (84.6%) | 252 (92.0%) | 188 (78.7%) |
| Number of critical incidents each year | 14.53 (20.76) | 30.77 (51.04) | 34.47 (52.54) |
| Exposed to personal life threat each year1 | 207 (62.4%) | 177 (64.6%) | 142 (59.4%) |
| Number of personal life threat events each year |
6.02 (14.44) | 8.85 (27.79) | 9.00 (26.01) |
| PDI | .58 (.50) | .67 (.54) | .68 (.56) |
| PDEQ | 1.40 (.54) | 1.49 (.63) | 1.45 (.57) |
| ASDS | 25.58 (7.44) | 27.02 (9.97) | 27.27 (10.86) |
| PCL | |||
| Continuous Score | 19.90 (5.69) | 19.71 (4.98) | 20.31 (6.32) |
| Partial PTSD | 7 (2.5%) | 8 (3.2%) | 4 (1.8%) |
| Full PTSD | 1 (0.4%) | 0 (0%) | 5 (2.3%) |
ASDS, Acute Stress Disorder Scale; PCL, PTSD Checklist; PDEQ, Peritraumatic Dissociative Experiences Questionnaire; PTSD, posttraumatic stress disorder.
Defined as exposure to one or more of 14 critical incidents that posed a direct threat to life or physical integrity (68)
3.2 Cortisol Awakening Response and Peritraumatic distress, Peritraumatic Dissociation, and PTSD Symptoms
Separate mixed model analyses were conducted for the four outcome variables (peritraumatic dissociation, peritraumatic distress, ASD symptoms and PTSD symptoms). Covariates included time of awakening (significantly associated with cortisol awakening variables (see table 3) and peritraumatic distress (r=−.15, p<.05)) and cumulative critical incident stress exposure assessed at each yearly follow up (significantly associated with PDI at 12 months (r=.19, p<.01) and 36 months (r=.17, p<.05), PDEQ at 12 months (r=.16, p<.05) and 24 months, (r =.18, p<.01), ASDS at 12 months, (r=.16, p<.05) and 36 months, (r=.22, p <.01), and PCL at 24 months: (r=.15, p<.05).
Table 3.
Pearson Correlations of Covariates, Cortisol Variables and Outcome Variables
| Awakening Cortisol Level |
Cortisol Awakening Response (Δ change) |
|
|---|---|---|
| Baseline | ||
| Age | .14** | −.04 |
| Smoking | −.06 | .02 |
| Wake time | .13* | −.12* |
| Prior Trauma | −.01 | .06 |
| Body Mass Index | −.04 | −.04 |
| 12 Months | ||
| PDI | −.13* | .09 |
| PDEQ | .00 | .13* |
| ASDS | −.13† | .19* |
| PCL | −.01 | −.02 |
| 24 Months | ||
| PDI | −.08 | .13† |
| PDEQ | −.12† | .13† |
| ASDS | −.06 | .14† |
| PCL | −.05 | .22** |
| 36 Months | ||
| PDI | .00 | .09 |
| PDEQ | −.01 | .21** |
| ASDS | .03 | .07 |
| PCL | .01 | .06 |
ASDS, Acute Stress Disorder Scale; PCL, PTSD Checklist; PDEQ, Peritraumatic Dissociative Experiences Questionnaire; PDI, Peritraumatic Distress Inventory; PTSD, posttraumatic stress disorder.
p < .10
p <.05
p < .01
Greater sleep difficulties during academy training assessed with the PSQI were associated with lower first awakening cortisol levels, r=−.11, p<.05, greater CAR, r=.13, p<.05, higher scores on the ASD scale (excluding the ASDS sleep item) at 12 (r = .25, p<.001) and 24 months (r=.24, p<.01) and higher PCL scores (excluding the PCL sleep item) at 24 (r=.22, p<.05), and 36 months (r=.20, p<.01). However, since awakening time and sleep quality were correlated, r=−.23, p<.001, we chose awakening time as a covariate in subsequent analyses because of prior literature on the association of wake time with CAR (37-40).As seen in Table 4, after controlling for covariates, awakening cortisol, and cumulative critical incident stress exposure for each year of service, greater cortisol awakening response during academy training was associated with greater average peritraumatic dissociation during the three year follow-up period, , β=.14, z=3.49, p<.0001. The model examining ASD symptoms (Table 4) indicated that greater cortisol awakening response was also associated with greater average ASD symptoms even after controlling for covariates, β=.09, z=2.03, p<.05. The models examining CAR as a predictor of peritraumatic distress and posttraumatic stress symptoms were not significant, β=.03, z=.81, p=.42 and β=−.004, z=−.09, p=.93, respectively.
Table 4.
Mixed Model Examining Cortisol Awakening Response and Peritraumatic Dissociation, Peritraumatic Distress, Acute Stress Disorder Symptoms, and Posttraumatic Stress Disorder Symptoms (n = 202 - 245)
| Peritraumatic Dissociation |
Peritraumatic Distress | Acute Stress Disorder Symptoms |
Posttraumatic Stress Disorder Symptoms |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | β | Z | p | B | β | Z | p | B | β | Z | p | B | β | Z | p | |
| Timepoint | .002 | .05 | 1.21 | .23 | .005 | .12 | 2.39 | .02 | .03 | .05 | .91 | .37 | .007 | .02 | .34 | .73 |
| Cumulative CI Exposure |
.0003 | .16 | 4.24 | .000 | .0003 | .19 | 5.00 | .000 | .006 | .19 | 4.25 | .000 | .003 | .17 | 4.04 | .000 |
| Time of Awakening |
−.01 | −.04 | −1.44 | .15 | −.01 | −.03 | −.93 | .35 | −.06 | −.02 | −.49 | .62 | −.18 | −.07 | −2.34 | .02 |
| Awakening Cortisol |
.08 | .07 | 1.71 | .09 | .02 | .02 | .52 | .61 | 1.29 | .07 | 1.41 | .16 | .48 | .05 | 1.37 | .17 |
| Cortisol Awakening Response |
.17 | .14 | 3.49 | .000 | .04 | .03 | .81 | .42 | 1.74 | .09 | 2.03 | .04 | −.04 | −.004 | −.09 | .93 |
CI = Critical Incident; β = Standardized Beta
4. Discussion
We found that a greater increase in salivary cortisol from 0 to 30 minutes after awakening, assessed during academy training, is a prospective predictor of greater peritraumatic dissociation and ASD symptoms across the first three years of police service. Our results suggest that a greater CAR, and not a lower CAR as we had originally predicted, is a pre-existing vulnerability factor for peritraumatic dissociation and ASD symptoms. To our knowledge, this is one of the first and largest prospective studies to evaluate the relationship between HPA axis activity prior to trauma exposure and the development of symptomatology over multiple years of follow-up.
Our results did not demonstrate a significant association between CAR at baseline and later PTSD symptoms, consistent with the study by Heinrichs and colleagues (23), despite the significantly larger sample size in our study (e.g., over 200 police officers compared to 43 firefighters). Our ability to detect a relationship may have been impacted by limited variability in PTSD symptoms, consistent with literature indicating that police officers and other emergency services personnel report low levels of PTSD early in their careers, although levels of symptomatology have been shown to increase over years of service (41-43). Reports of low symptoms may also be due to self-selection for emergency work, rigorous screening prior to acceptance into the police academy, and officers’ stigma related minimization of symptoms (41, 42, 44). For that reason we also examined peritraumatic reactivity and ASD symptoms which have greater variability early in service, may be less reactive to stigma concerns as they are regarded by officers as expectable responses to police operational stressors, and may foreshadow the later development of PTSD (3, 5, 45). It will be important to reexamine these relationships in the future when this cohort has experienced a greater accumulation of critical incident stress exposure. In addition, replication in civilians, using a prospective design, will be important for establishing generalizability of our findings.
Several longitudinal studies examining cortisol levels shortly after trauma found significant associations with the later development of PTSD symptoms. For example, higher initial urinary cortisol (collected over 15 hours after the event) predicted later PTSD symptoms in traumatized children (45). However, two studies of adults found that lower initial urinary or plasma cortisol levels in the immediate aftermath of a traumatic event predicted posttraumatic symptoms (21, 22) while another study failed to find a relationship (20). Additional analyses by these investigators have suggested that prior trauma histories may have accounted for correlations between initial cortisol levels and PTSD symptoms (46, 47). However, we did not find a significant relationship between prior trauma and CAR in our study.
Our finding of greater CAR during academy training predicting greater peritraumatic dissociation and ASD symptoms may indicate greater reactivity to acute stress in certain officers. Presumably, recruits who react to awakening with greater increases in cortisol will also be more reactive to stress during academy training and to critical incident stressors when they occur during police service. A possible mechanism that could underlie greater stress reactivity across time is excessive amygdala activation with insufficient emotion modulation by the prefrontal cortex. An imbalance in activation of these areas has been suggested to account for greater and more prolonged states of fear driven activation (e.g., 6, 7), which presumably results in greater fear conditioning and overconsolidation of trauma memory, leading to chronic hyperarousal symptoms seen in ASD and PTSD (8).
However, since we did not capture real time peritraumatic biological stress responses to critical incidents, we can only speculate on possible linking mechanisms. Increased cortisol release has been shown to enhance memory consolidation of emotionally stressful material and this may be a mechanism that leads to overconsolidation of traumatic memories (48). It has also been suggested that glucocorticoids can potentiate some of the actions of catecholamines (49-51), which also includes memory consolidation of emotionally arousing material (52). Alternatively, Yehuda (9) has argued that lower cortisol reactivity during trauma exposure may be a risk factor for the development of PTSD, since cortisol may constrain the sympathetic response. Since the sympathetic nervous system is also involved in the switch to greater adrenal sensitivity to ACTH associated with awakening, it may account for both greater CAR and greater stress reactivity during the peritraumatic period. Future studies examining both the CAR and more immediate sympathetic and HPA axis responses in the peritraumatic period may be able to address questions of mechanisms more directly. While the role of the CAR in dissociation and ASD is not fully understood, one important function of the CAR is believed to be the ending of sleep inertia and the facilitation of alertness upon awakening (53). In the present study, we found a relationship between greater sleep difficulties during academy training and greater CAR, peritraumatic reactivity, ASD and PTSD symptoms, consistent with some, but not all, (54-56) previous reports of short sleep duration and disturbed sleep associated with indices of higher awakening HPA axis activity (57, 58). Previous studies suggest that Circadian factors likely affect the CAR. For example, the CAR following daytime sleep is smaller than collections after nighttime sleep (59), although controlling for wake time did not diminish our significant findings. The HPA axis may play a causal role in sleep disturbance and also may be altered as a result of sleep disturbances (60). For instance, HPA axis hyperreactivity is believed to contribute to insomnia and corticotropin releasing factor (CRF) administration has been associated with increased time awake (61, 62). However, a study of repeated forced wakings found no effect on CAR (63), suggesting the possibility of other contributory factors.
One possible candidate is acute stress reactivity which is suggested by previous findings that those with shorter sleep duration rated life events that they experienced the previous day as more stressful (57). Sleep deprivation has also been identified as a stressor contributing to allostatic load (64), thus, poorer sleep quality may contribute to the experience or perception of chronic stress. While our initial focus was on the relationship between CAR and peritraumatic and posttraumatic stress responding, the strength of the relationship of our variables of interest with poorer sleep quality was unexpectedly consistent. While these variables are likely to be interrelated and the direction of causality is difficult to determine, these findings point to an important area for further study that we will be able to examine more carefully with the addition of objective markers of sleep that are currently being collected prospectively in this cohort.
It is important to note that measures of CAR often differ across studies. Previous studies examining PTSD have typically used composite measures of awakening cortisol (e.g., area under the curve with respect to the ground/zero (AUCG) or mean values across the first hour of awakening). A limitation of that approach is that it does not differentiate between integrated levels of cortisol released over the awakening period, and includes the circadian rise in cortisol in the morning, pre-awakening influences and the dynamic increase in cortisol related to mobilizing arousal pathways associated with the wake state (53). In the present study, we examined the change from 0 to 30 minutes after awakening and included cortisol awakening levels in our statistical analysis as suggested by Clow and colleagues (53). This method provides two distinguishable measures (e.g., the end state of pre-awakening cortisol secretion and the dynamic increase of the response) that result from different regulatory mechanisms. Further refinements in measurement of the components of cortisol over the awakening period would allow us to more precisely model mechanisms that may predict symptoms.
An important limitation of our study is that cortisol is sampled only twice, at 0 and 30 minutes after awakening. This sampling scheme does not capture the return to baseline. Previous research has indicated substantial individual differences in recovery that is evident even after 60 minutes of sampling (12); however, the functional significance of sustained elevated levels on target tissues is not known. Furthermore, future studies should consider sampling cortisol across multiple days, controlling for day-to-day individual variability in cortisol awakening responses and state psychological measures. Studies have shown that multiple days of sampling provides a more reliable trait-like estimate of HPA activity that is less vulnerable to state influences (65, 66). It is also plausible that other unmeasured psychosocial or contextual factors could modulate the relationship between CAR and symptom expression and this possibility warrants future study. Additionally, we relied on self-reported adherence to instructions for cortisol sampling rather than digitized monitoring of saliva sampling relative to wake-up times. A delay of >15 minutes between awakening and first cortisol collection has been shown to result in smaller CAR values and can confound findings (67). Unwanted variability due to these measurement issues may have contributed to our inability to detect an association between CAR and PTSD symptoms. In addition, it is important to consider that non-adherence to the sampling protocol may vary systematically with stress or psychiatric symptoms and contribute to significant associations which would be artifacts.
Despite these limitations, the present study is the largest to date that prospectively evaluates the relationships among CAR assessed prior to trauma exposure, and the subsequent development of peritraumatic, acute, and chronic posttraumatic responses; and the first to report increased CAR as a candidate pre-existing risk factor for the development of ASD symptoms, supporting a model of pre-exposure differences in HPA axis activity as a marker for vulnerability. Whether this relationship also accounts for the development of PTSD has yet to be determined in future large prospective studies with more symptomatic populations and mechanisms underlying these relationships have yet to be delineated.
Figure 1.
Mean cortisol concentrations for individuals scoring in the top and bottom quartiles on peritraumatic dissociation (PDEQ). The association between the cortisol awakening response at baseline and PDEQ scores at 12, 24, 36 months was found to be significant in a covariate adjusted mixed model, p<.0001.
Figure 2.
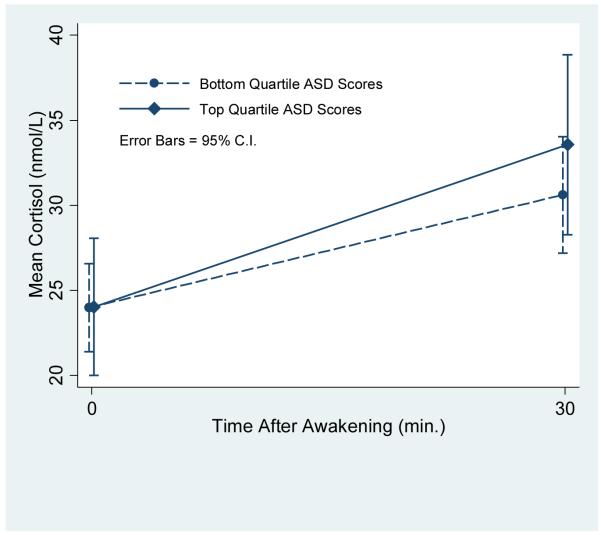
Mean cortisol concentrations for individuals scoring in the top and bottom quartiles on the Acute Stress Disorder Scale. The association between the cortisol awakening response at baseline and ASD scores at 12, 24, and 36 months was found to be significant in a covariate adjusted mixed model, p<.05.
Acknowledgements
This research was supported by National Institute of Mental Health grant (R01 – MH056350-06) to Dr. Marmar. Dr. Inslicht and Dr. McCaslin were supported by VA Clinical Science Research and Development, Career Development Awards (CDA-2-037-07F and CDA-2-032-06F, respectively). We thank the police cadets who volunteered their time to participate in this research, as well as Suzanne Best, Ph.D., Marie-Hélène St-Hilaire, Ph.D., and Aoife O’Donovan, Ph.D., for their scientific contributions, and Maryann Lenoci and Gary Tarasovsky for their assistance in the collection and management of these data.
Footnotes
The appearance of baseline cortisol both as part of the calculation of CAR and as a covariate in its own right represents a re-parameterization of a simpler model in which baseline and Time 2 cortisol are entered together. The re-parameterization yields parameters that are more easily interpretable as the effects of CAR adjusted for baseline response, rather than the effects of Time 2 cortisol adjusted for baseline response.
Financial Disclosures The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brunet A, Weiss DS, Metzler TJ, Best SR, Neylan TC, Rogers C, et al. The Peritraumatic Distress Inventory: a proposed measure of PTSD criterion A2. Am J Psychiatry. 2001;158:1480–1485. doi: 10.1176/appi.ajp.158.9.1480. [DOI] [PubMed] [Google Scholar]
- 2.Cardena E, Spiegel D. Dissociative reactions to the San Francisco Bay Area earthquake of 1989. Am J Psychiatry. 1993;150:474–478. doi: 10.1176/ajp.150.3.474. [DOI] [PubMed] [Google Scholar]
- 3.Marmar CR, Metzler TJ, Otte C. The Peritraumatic Dissociative Experiences Questionnaire. In: Wilson J, Keane T, editors. Assessing Psychological Trauma and PTSD. 2 ed Guilford; New York: 2003. pp. 144–167. [Google Scholar]
- 4.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth ed American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 5.Bryant RA. Early predictors of posttraumatic stress disorder. Biol Psychiatry. 2003;53:789–795. doi: 10.1016/s0006-3223(02)01895-4. [DOI] [PubMed] [Google Scholar]
- 6.Pitman RK, Shin LM, Rauch SL. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry. 2001;62(Suppl 17):47–54. [PubMed] [Google Scholar]
- 7.Bremner JD. Neuroimaging studies in post-traumatic stress disorder. Curr Psychiatry Rep. 2002;4:254–263. doi: 10.1007/s11920-996-0044-9. [DOI] [PubMed] [Google Scholar]
- 8.Pitman R, Shalev AY, Orr SP. Posttraumatic stress disorder: Emotion, conditioning, and memory. In: Corbetta M, Gazzaniga M, editors. The new cognitive neurosciences. Plenum Press; New York: 2000. pp. 687–700. [Google Scholar]
- 9.Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- 10.Dallman MF, Bhatnager S, Viau V. Hypothalamo-pituitary-adrenal axis. In: Fink G, editor. Encyclopedia of Stress. vol 2. Academic Press; San Diego: 2000. pp. 468–476. [Google Scholar]
- 11.Edwards S, Clow A, Evans P, Hucklebridge F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci. 2001;68:2093–2103. doi: 10.1016/s0024-3205(01)00996-1. [DOI] [PubMed] [Google Scholar]
- 12.Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response - normal values and confounds. Noise Health. 2000;2:79–88. [PubMed] [Google Scholar]
- 13.Schlotz W, Hellhammer J, Schulz P, Stone AA. Perceived work overload and chronic worrying predict weekend-weekday differences in the cortisol awakening response. Psychosom Med. 2004;66:207–214. doi: 10.1097/01.psy.0000116715.78238.56. [DOI] [PubMed] [Google Scholar]
- 14.Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience--cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci U S A. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lauc G, Zvonar K, Vuksic-Mihaljevic Z, Flogel M. Short Communication: Post-awakening changes in salivary cortisol in veterans with and without PTSD. Stress and Health. 2004;20:99–102. [Google Scholar]
- 16.Wessa M, Rohleder N, Kirschbaum C, Flor H. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:209–215. doi: 10.1016/j.psyneuen.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Neylan TC, Brunet A, Pole N, Best SR, Metzler TJ, Yehuda R, et al. PTSD symptoms predict waking salivary cortisol levels in police officers. Psychoneuroendocrinology. 2005;30:373–381. doi: 10.1016/j.psyneuen.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Johnson DM, Delahanty DL, Pinna K. The cortisol awakening response as a function of PTSD severity and abuse chronicity in sheltered battered women. J Anxiety Disord. 2008;22:793–800. doi: 10.1016/j.janxdis.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simeon D, Yehuda R, Knutelska M, Schmeidler J. Dissociation versus posttraumatic stress: cortisol and physiological correlates in adults highly exposed to the World Trade Center attack on 9/11. Psychiatry Res. 2008;161:325–329. doi: 10.1016/j.psychres.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Shalev AY, Videlock EJ, Peleg T, Segman R, Pitman RK, Yehuda R. Stress hormones and post-traumatic stress disorder in civilian trauma victims: a longitudinal study. Part I: HPA axis responses. Int J Neuropsychopharmacol. 2008;11:365–372. doi: 10.1017/S1461145707008127. [DOI] [PubMed] [Google Scholar]
- 21.Resnick HS, Yehuda R, Acierno R. Acute post-rape plasma cortisol, alcohol use, and PTSD symptom profile among recent rape victims. Ann N Y Acad Sci. 1997;821:433–436. doi: 10.1111/j.1749-6632.1997.tb48298.x. [DOI] [PubMed] [Google Scholar]
- 22.Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol Psychiatry. 2000;48:940–947. doi: 10.1016/s0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- 23.Heinrichs M, Wagner D, Schoch W, Soravia LM, Hellhammer DH, Ehlert U. Predicting posttraumatic stress symptoms from pretraumatic risk factors: a 2-year prospective follow-up study in firefighters. Am J Psychiatry. 2005;162:2276–2286. doi: 10.1176/appi.ajp.162.12.2276. [DOI] [PubMed] [Google Scholar]
- 24.Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin North Am. 2002;25:341–368. doi: 10.1016/s0193-953x(02)00002-3. [DOI] [PubMed] [Google Scholar]
- 25.Derogatis LR. SCL-90-R: Administration scoring and procedures manual 1. Clinical Psychometrics Research; Baltimore, MD: 1997. [Google Scholar]
- 26.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis 1 Disorders - Non-patient Edition (SCID-I/NP, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1996. [Google Scholar]
- 27.Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57:675–682. doi: 10.1001/archpsyc.57.7.675. [DOI] [PubMed] [Google Scholar]
- 28.Derogatis LR. SCL-90-R administration, scoring, and procedures manual. 3rd ed National Computer Systems, Inc.; Minneapolis: 1994. [Google Scholar]
- 29.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe J, Kimerling R, Brown PJ, Chresman KR, Levin K. Psychometric review of the life stressor checklist-revised. Sidran Press; Lutherville, MD: 1996. [Google Scholar]
- 31.Otte C, Neylan TC, Pole N, Metzler T, Best S, Henn-Haase C, et al. Association between childhood trauma and catecholamine response to psychological stress in police academy recruits. Biol Psychiatry. 2005;57:27–32. doi: 10.1016/j.biopsych.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: An overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- 33.Steptoe A, Brydon L, Kunz-Ebrecht S. Changes in financial strain over three years, ambulatory blood pressure, and cortisol responses to awakening. Psychosom Med. 2005;67:281–287. doi: 10.1097/01.psy.0000156932.96261.d2. [DOI] [PubMed] [Google Scholar]
- 34.Weiss DS, Brunet A, Best SR, Metzler TJ, Liberman A, Pole N, et al. Frequency and severity approaches to indexing exposure to trauma: The Critical Incident History Questionnaire for police officers. J Trauma Stress. 2010;23:734–743. doi: 10.1002/jts.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruggiero KJ, Del Ben K, Scotti JR, Rabalais AE. Psychometric properties of the PTSD Checklist-Civilian Version. J Trauma Stress. 2003;16:495–502. doi: 10.1023/A:1025714729117. [DOI] [PubMed] [Google Scholar]
- 36.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behav Res Ther. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 37.Stalder T, Hucklebridge F, Evans P, Clow A. Use of a single case study design to examine state variation in the cortisol awakening response: Relationship with time of awakening. Psychoneuroendocrinology. 2009;34:607–614. doi: 10.1016/j.psyneuen.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 38.Edwards S, Clow A, Evans P, Hucklebridge F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci. 2001;68:2093–2103. doi: 10.1016/s0024-3205(01)00996-1. [DOI] [PubMed] [Google Scholar]
- 39.Dahlgren A, Kecklund G, Theorell T, Akerstedt T. Day-to-day variation in saliva cortisol—relation with sleep, stress and self-rated health. Biol Psychol. 2009;82:149–155. doi: 10.1016/j.biopsycho.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28:35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- 41.Bryant RA, Guthrie RM. Maladaptive self-appraisals before trauma exposure predict posttraumatic stress disorder. J Consult Clin Psychol. 2007;75:812–815. doi: 10.1037/0022-006X.75.5.812. [DOI] [PubMed] [Google Scholar]
- 42.Bryant RA, Guthrie RM. Maladaptive appraisals as a risk factor for posttraumatic stress: A study of trainee firefighters. Psychol Sci. 2005;16:749–752. doi: 10.1111/j.1467-9280.2005.01608.x. [DOI] [PubMed] [Google Scholar]
- 43.Pole N. Predictors of PTSD symptoms in police officers: From childhood to retirement. In: Delahanty DL, editor. The Psychobiology of Trauma and Resilience Across the Lifespan. Jason Aronson; Lanham, MD: 2008. pp. 47–67. [Google Scholar]
- 44.Ozer EJ, Best SR, Lipsey TL, Weiss DL. Predictors of posttraumatic stress disorder and symptoms in adults: A meta-analysis. Psychol Bull. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- 45.Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology. 2005;30:121–128. doi: 10.1016/j.psyneuen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Yehuda R, Resnick HS, Schmeidler J, Yang RK, Pitman RK. Predictors of cortisol and 3-methoxy-4-hydroxyphenylglycol responses in the acute aftermath of rape. Biol Psychiatry. 1998;43:855–859. doi: 10.1016/s0006-3223(97)00554-4. [DOI] [PubMed] [Google Scholar]
- 47.Delahanty DL, Raimonde AJ, Spoonster E, Cullado M. Injury severity, prior trauma history, urinary cortisol levels, and acute PTSD in motor vehicle accident victims. J Anxiety Disord. 2003;17:149–164. doi: 10.1016/s0887-6185(02)00185-8. [DOI] [PubMed] [Google Scholar]
- 48.Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- 49.Gibson A. The influence of endocrine hormones on the autonomic nervous system. J Auton Pharmacol. 1981;1:331–358. doi: 10.1111/j.1474-8673.1981.tb00463.x. [DOI] [PubMed] [Google Scholar]
- 50.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 51.Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: An update and integration. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2011.07.002. (in press) [DOI] [PubMed] [Google Scholar]
- 52.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 53.Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: More than a measure of HPA axis function. Neurosci Biobehav Rev. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Backhaus J, Junghanns K, Hohagen F. Sleep disturbances are correlated with decreased morning awakening salivary cortisol. Psychoneuroendocrinology. 2004;9:1184–1191. doi: 10.1016/j.psyneuen.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 55.Waye KP, Clow A, Edwards S, Hucklebridge F, Rylander R. Effects of nighttime low frequency noise on the cortisol response to awakening and subjective sleep quality. Life Sci. 2003;72:863–875. doi: 10.1016/s0024-3205(02)02336-6. [DOI] [PubMed] [Google Scholar]
- 56.Lasikiewicz N, Hendrickx H, Talbot D, Dye L. Exploration of basal diurnal salivary cortisol profiles in middle-aged adults: associations with sleep quality and metabolic parameters. Psychoneuroendocrinology. 2008;33:143–151. doi: 10.1016/j.psyneuen.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 57.Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009;94:4801–4809. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okun ML, Krafty RT, Buysse DJ, Monk TH, Reynolds CF, 3rd, Begley A, Hall M. What constitutes too long of a delay? Determining the cortisol awakening response (CAR) using self-report and PSG-assessed wake time. Psychoneuroendocrinology. 2009;35:460–468. doi: 10.1016/j.psyneuen.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griefahn B, Robens S. The cortisol awakening response: A pilot study on the effects of shift work, morningness and sleep duration. Psychoneuroendocrinology. 2008;33:981–988. doi: 10.1016/j.psyneuen.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: Normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- 61.Ehlers CL, Reed TK, Hendrikson SJ. Effects of corticotropin-releasing factor and growth hormone-releasing factor on sleep and activity in rats. Neuroendocrinology. 1986;42:467–474. doi: 10.1159/000124489. [DOI] [PubMed] [Google Scholar]
- 62.Chang FC, Opp MR. Corticotropin-releasing hormone (CRH) as a regulator of waking. Neurosci Biobehav Rev. 2001;25:445–453. doi: 10.1016/s0149-7634(01)00024-0. [DOI] [PubMed] [Google Scholar]
- 63.Dettenborn L, Rosenloecher F, Kirschbaum C. No effects of repeated forced wakings during three consecutive nights on morning cortisol awakening responses (CAR): A preliminary study. Psychoneuroendocrinology. 2007;32:915–921. doi: 10.1016/j.psyneuen.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 64.McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism. 2006;55:S20–S23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 65.Adam EK, Kumari M. Assessing salivary cortisol in largescale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 66.Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 67.Okun ML, Krafty RT, Buysse DJ, Monk TH, Reynolds CF, 3rd, Begley A, Hall M. What constitutes too long of a delay? Determining the cortisol awakening response (CAR) using self-report and PSG-assessed wake time. Psychoneuroendocrinology. 2010;35:460–468. doi: 10.1016/j.psyneuen.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCaslin SE, Rogers CE, Metzler TJ, Best SR, Weiss DS, Fagan JA, et al. The impact of personal threat on police officers’ responses to critical incident stressors. J Nerv Ment Dis. 2006;194:591–597. doi: 10.1097/01.nmd.0000230641.43013.68. [DOI] [PubMed] [Google Scholar]