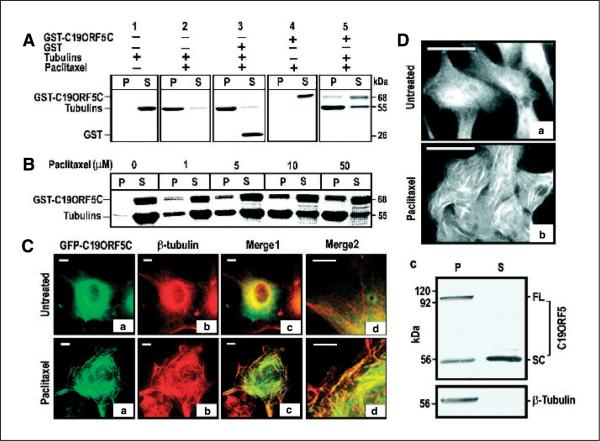
Figure 2.
Specific association of the C19ORF5 with paclitaxel-stabilized microtubules. A, association of purified GST-C19ORF5C with paclitaxel-stabilized microtubules. Tubulins (10 μg) were incubated with 10 μg GST-C19ORF5C, GST, or other control GST fusion proteins in G-PEM buffer for 30 minutes at 37°C in the presence of 5 μmol/L paclitaxel. The 20 μL mixtures were layered on 10 μL of 10% sucrose solution in PEM buffer and centrifuged at 10,000 × g for 20 minutes at room temperature. Supernatant (S, 30 μL) containing soluble tubulins and other relatively low molecular weight components and pellet (P) resuspended in 30 μL G-PEM buffer containing stabilized microtubules and associated proteins were loaded side by side on 7.5% SDS-PAGE. Protein bands were visualized with Coomassie blue stain. B, paclitaxel concentration-dependent association of GST-C19ORF5C protein with microtubules. Microtubules were assembled in the presence of the same amount of GST-C19ORF5C and tubulin employed in (A) with the indicated amounts of paclitaxel. C, specific association of the C19ORF5 with paclitaxel-stabilized microtubules in vivo. COS7 cells treated with 10 μmol/L paclitaxel where indicated at the time of transfection with cDNA coding for GFP-C19ORF5C were stained with anti-β-tubulin and Texas red–conjugated anti-mouse antibody. The relationship of GFP (green) and anti-β-tubulin antibody (red) fluorescence was observed and colors merged to test for overlap of signals (yellow). Merge2, segments of cells in Merge1 magnified 2.5 times. Representative of 90% of transfected cells from three independent experiments. D, distribution of native C19ORF5 in normal (a) and paclitaxel-treated HepG2 cells (b) and its association with paclitaxel-stabilized microtubules from HepG2 cell lysate in vitro (c). Cells were treated with paclitaxel as indicated and analyzed with mAb4G1. Representative of the majority of cells in three independent experiments. For in vitro assembly of microtubules, ~1 × 106 HepG2 cells were lysed in 150 μL buffer and followed by sonication for 10 seconds. The cell lysate was clarified by centrifugation at 10,000 × g for 20 minutes and treated with 10 μmol/L paclitaxel at 37°C for 1 hour to stabilize microtubules and the reaction mixture was loaded on a cushion of 20 μL of 20% sucrose. Stabilized microtubules and associated proteins were sedimented by ultracentrifugation (Sorvall RC M120) at 100,000 × g for 1 hour. The pellet containing the stabilized microtubules was resuspended in 150 μL buffer. An equal volume of pellet and supernatant (25 μL) fractions were analyzed on SDS-PAGE and immunoblotted with antibodies against C19ORF5 (mAb4G1) or β-tubulin, respectively. FL, full-length C19ORF5 predicted from cDNA; SC, short chain.