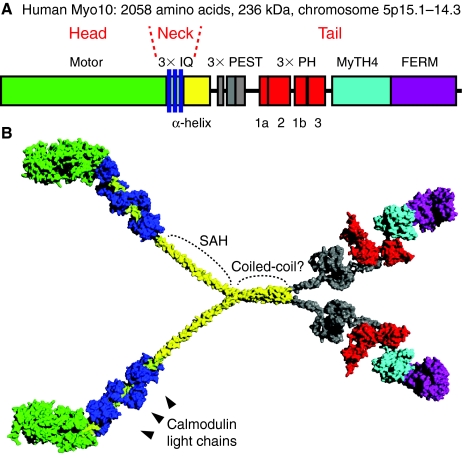
Fig. 1.
Structure of Myo10. (A) Diagram showing the domain structure of the Myo10 heavy chain. The cluster of three PH domains has a somewhat unusual arrangement, with PH2 located in a surface loop of PH1, splitting PH1 into two segments (1a and 1b). (B) Hypothetical model of Myo10 as a dimer with each heavy chain bound to three calmodulin light chains (dark blue). Note that other than the recently reported structures of the MyTH4-FERM region, the structure and overall organization of the Myo10 molecule remains unclear. The model here was constructed in PyMol on the basis of homology to domains of known structure to illustrate the relative size of the different domains. The neck is expected to have a length of ~11 nm. The beginning of the tail consists of a ~130-amino-acid α-helical region (yellow) that is depicted here consisting of a combination of an initial segment of single α-helix (SAH) and a distal segment of α-helical coiled-coil. Note that the precise length of the SAH is unclear and that the entire α-helical region is sometimes referred to as ‘CC’ because of initial predictions that it formed a coiled-coil. Although Myo10 is illustrated here as a parallel dimer, an antiparallel configuration has not been ruled out. The structure of the ~200-amino-acid region containing the three PEST sequences (gray) is completely unknown, but is depicted here as being relatively compact.