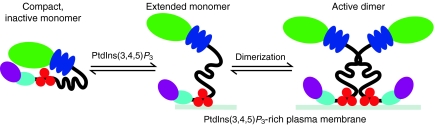
Fig. 4.
Model for Myo10 regulation. The tail of Myo10 binds to its head domain, forming a compact monomer that is enzymatically inactive (Umeki et al., 2011). Binding of the Myo10 tail to membranes containing PtdIns(3,4,5)P3 or PtdIns(4,5)P2 disrupts the intramolecular interactions between the head and tail, inducing the formation of an extended monomer. This, plus the increased local concentration of Myo10 resulting from its recruitment to membranes, is thought to lead to the formation of an active dimer that can move processively along actin.