Figure 3. Bacteroides’ primitive electron transport chain.
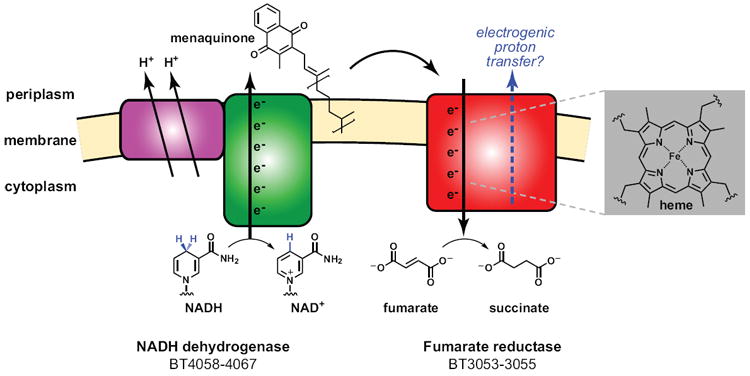
A proposed model for the anaerobic electron transport chain common to many Bacteroides species is shown; this model is based on crystal structures of NADH dehydrogenase and fumarate reductase from other bacteria (Efremov et al., 2010; Lancaster et al., 1999). The chain would consist of two enzyme complexes: a proton-translocating NADH-quinone oxidoreductase (NADH dehydrogenase, left) and a quinol:fumarate reductase (fumarate reductase, right). The action of NADH dehydrogenase would shuttle electrons from the oxidation of NADH to NAD+ down a pathway involving a flavin cofactor and multiple iron-sulfur clusters, ultimately reducing a membrane-bound menaquinone. This menaquinone would carry the electrons to fumarate reductase, which would shuttle them down a pathway involving one or two heme cofactors, possibly a second quinone, multiple iron-sulfur clusters, and a flavin cofactor that would reduce the terminal electron acceptor fumarate to succinate. ‘BT-numerical’ labels are the locus tags for genes within the B. thetatiotaomicron (VPI-5482) genome.
