Abstract
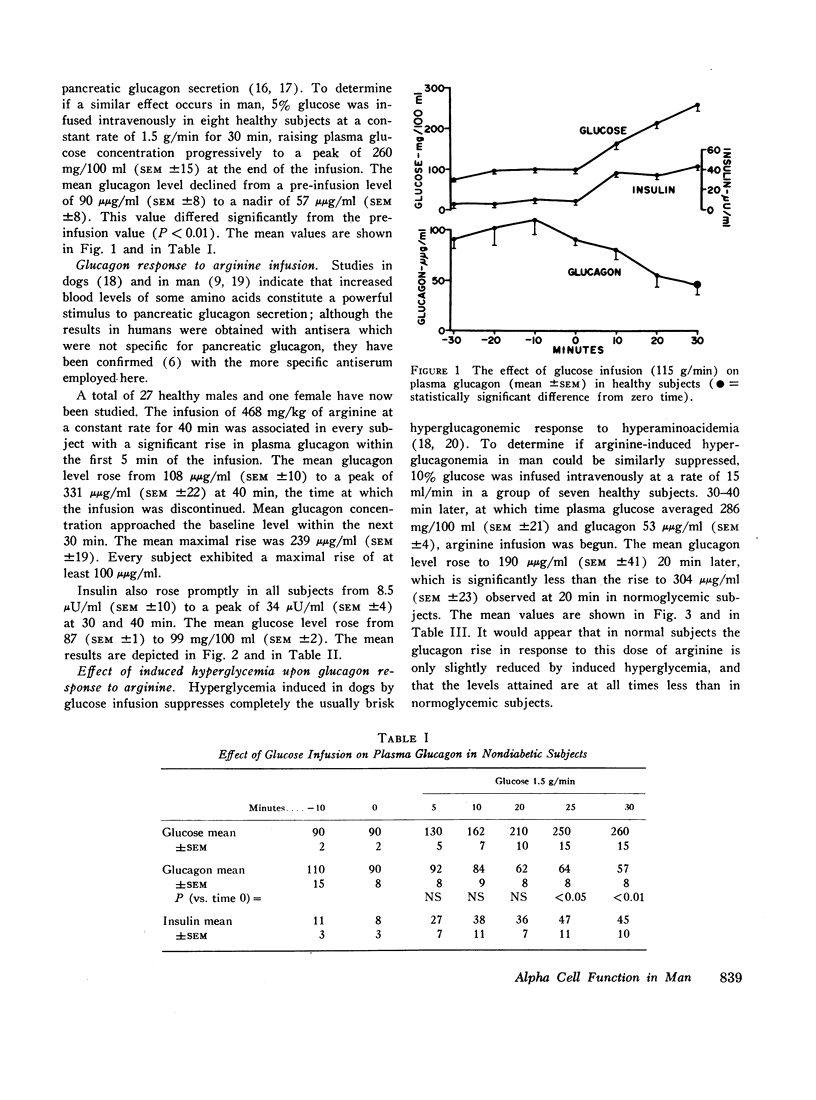
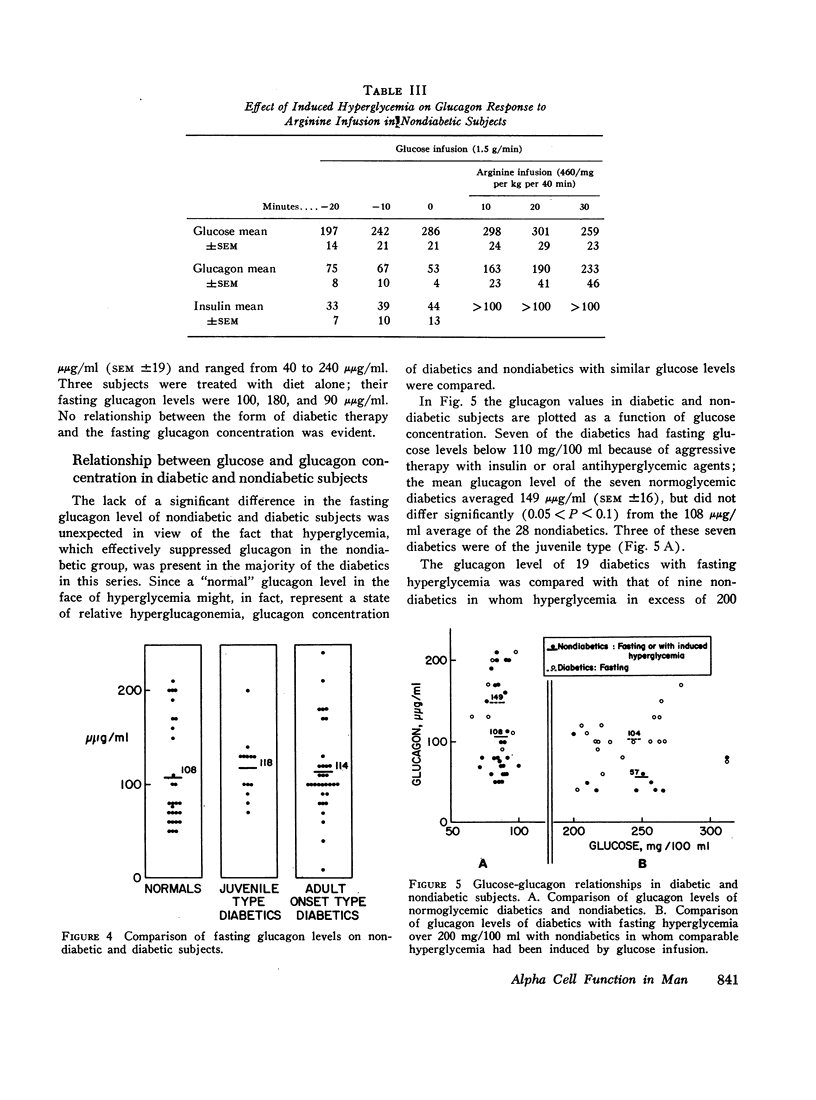
The development of a glucagon radioimmunoassay with a relatively high degree of specificity for pancreatic glucagon made possible studies of alpha cell function in healthy nondiabetic subjects and in patients with diabetes mellitus. In the former group mean fasting plasma glucagon averaged 108 μμg/ml (SEM ±10). In 12 juvenile-type diabetics fasting glucagon averaged 110 (±9) and in 33 adult-type diabetics the average was 114 (±8). The diabetic averages did not differ significantly from the nondiabetic subjects; however, when hyperglycemia was induced by glucose infusion in the nondiabetic subjects so as to simulate the fasting hyperglycemia of the diabetics, mean glucagon fell to 57 μμg (±8), which was significantly below the diabetic mean.
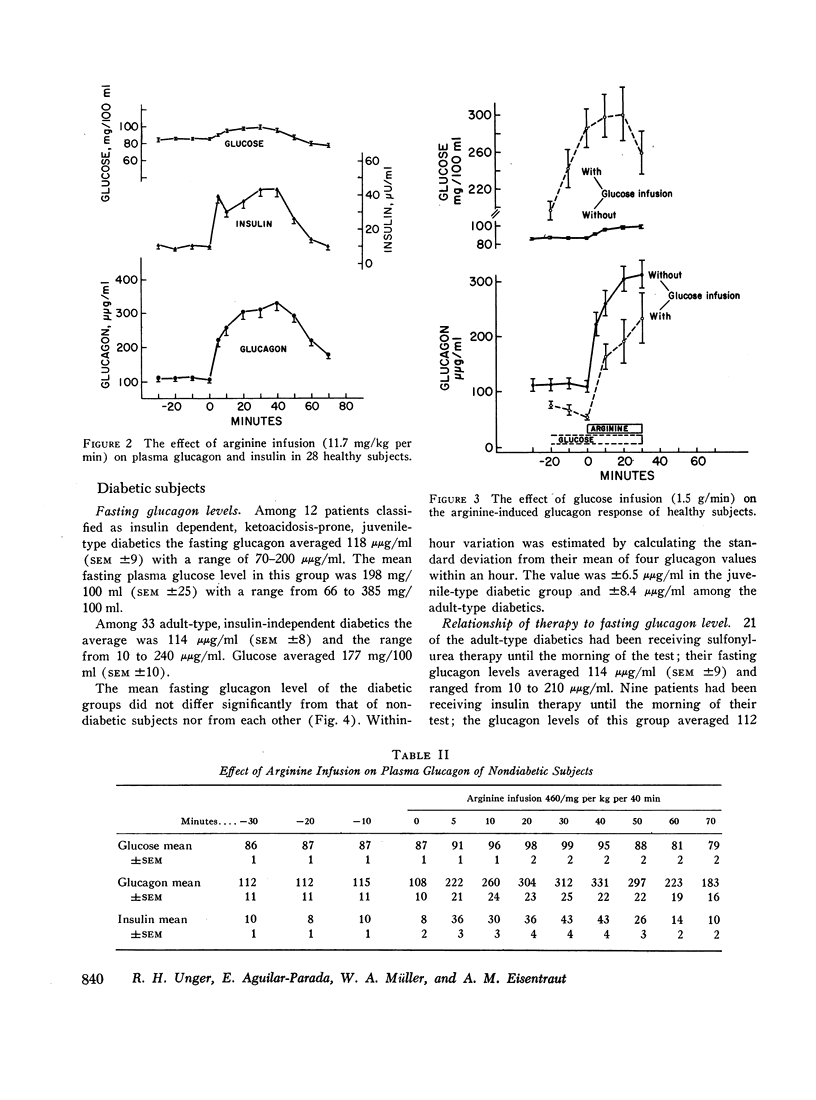
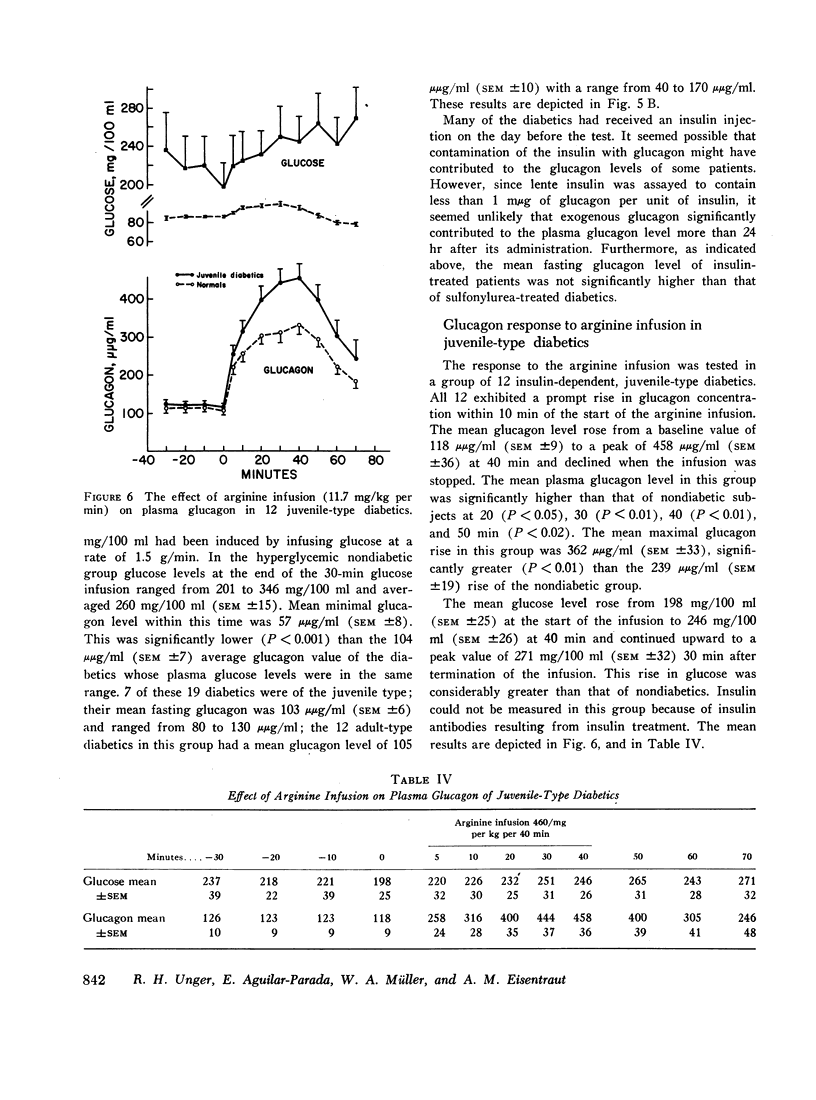
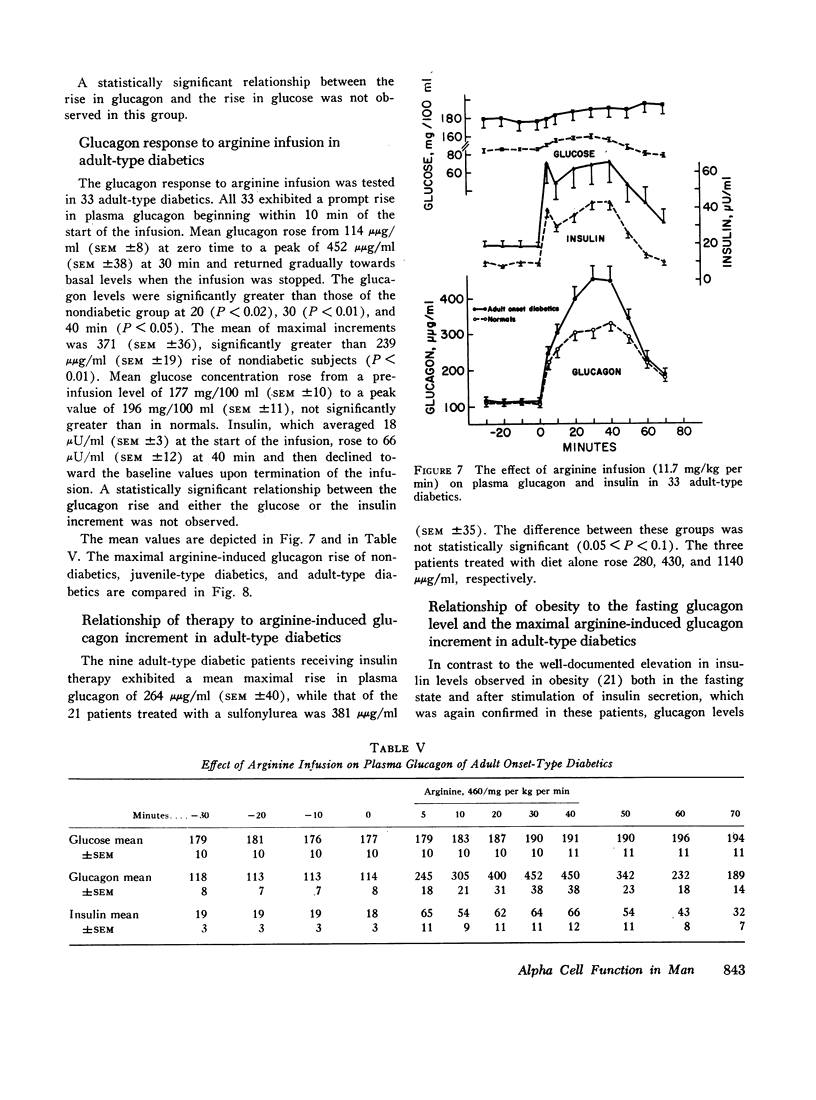
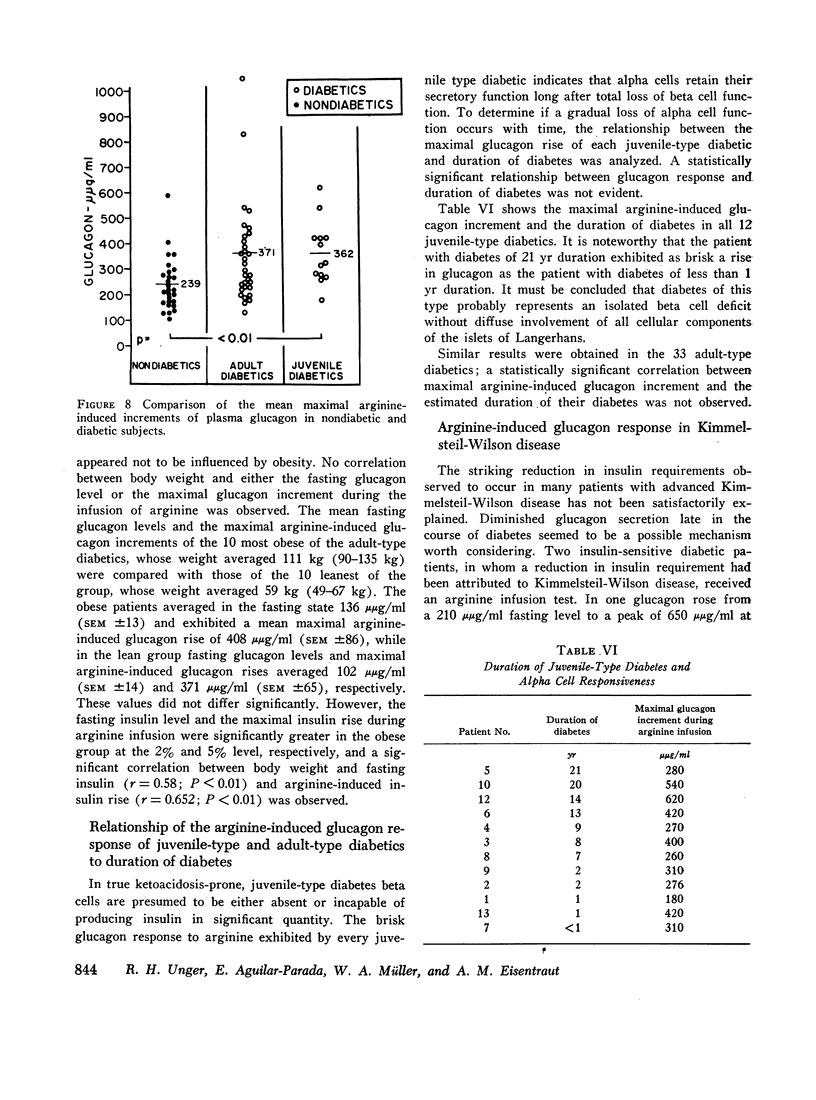
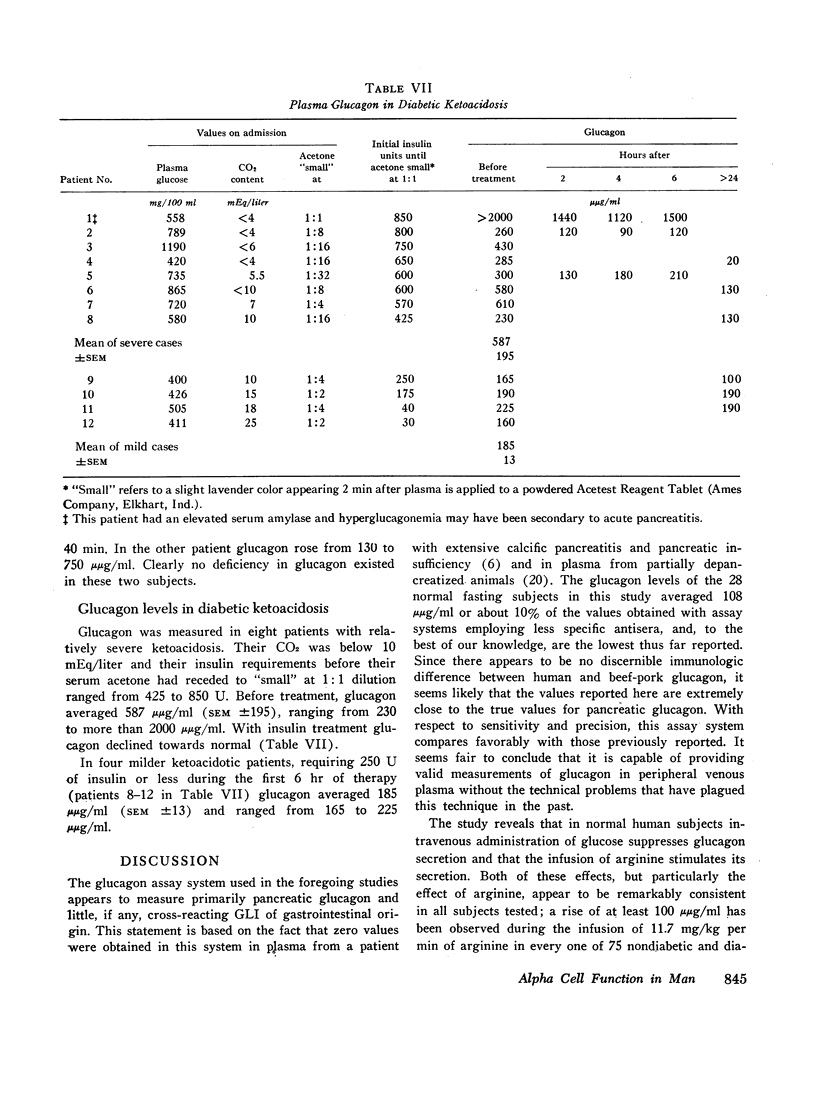
In 28 healthy subjects the infusion of arginine elicited a rise in glucagon of at least 100 μμg/ml with a peak level averaging 331 μμg/ml (±22) at 40 min. This response to arginine was diminished but not abolished during hyperglycemia induced by simultaneous glucose infusion. In everyone of 45 diabetic subjects tested the infusion of arginine elicited a rise in glucagon of at least 140 μμg/ml to levels significantly greater than in nondiabetics. The peak glucagon level in juvenile-type diabetics averaged 458 μμg/ml (SEM ±36) and in adult-type diabetics averaged 452 μμg/ml (SEM ±38). The glucagon response to arginine was unrelated to duration of diabetes, to body weight, type of diabetic treatment, or to other known factors. Marked hyperresponsiveness of glucagon to arginine infusion was observed in two patients with advanced Kimmelsteil-Wilson disease. Glucagon levels were markedly elevated in certain patients with severe diabetic ketoacidosis before treatment with insulin.
The findings suggest that alpha cell function is inappropriately increased in diabetes mellitus and could play a significant role in the diabetic syndrome.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Effects of starvation on plasma pancreatic glucagon in normal man. Diabetes. 1969 Nov;18(11):717–723. doi: 10.2337/diab.18.11.717. [DOI] [PubMed] [Google Scholar]
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969 Jun;257(6):415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- Charnley J., Eftekhar N. Penetration of gown material by organisms from the surgeon's body. Lancet. 1969 Jan 25;1(7587):172–173. doi: 10.1016/s0140-6736(69)91188-x. [DOI] [PubMed] [Google Scholar]
- FERNER H. The A- and B-cells of the pancreatic islets as sources of the antagonistic hormones glucagon and insulin; the shift of the AB-relation in diabetes mellitus. Am J Dig Dis. 1953 Oct;20(10):301–306. doi: 10.1007/BF02895538. [DOI] [PubMed] [Google Scholar]
- Fajans S. S., Floyd J. C., Jr, Knopf R. F., Conn F. W. Effect of amino acids and proteins on insulin secretion in man. Recent Prog Horm Res. 1967;23:617–662. doi: 10.1016/b978-1-4831-9826-2.50017-9. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- KARAM J. H., GRODSKY G. M., FORSHAM P. H. Excessive insulin response to glucose in obese subjects as measured by immunochemical assay. Diabetes. 1963 May-Jun;12:197–204. doi: 10.2337/diab.12.3.197. [DOI] [PubMed] [Google Scholar]
- Lawrence A. M. Radioimmunoassayable glucagon levels in man: effects of starvation, hypoglycemia, and glucose administration. Proc Natl Acad Sci U S A. 1966 Feb;55(2):316–320. doi: 10.1073/pnas.55.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohneda A., Aguilar-Parada E., Eisentraut A. M., Unger R. H. Control of pancreatic glucagon secretion by glucose. Diabetes. 1969 Jan;18(1):1–10. doi: 10.2337/diab.18.1.1. [DOI] [PubMed] [Google Scholar]
- Ohneda A., Parada E., Eisentraut A. M., Unger R. H. Characterization of response of circulating glucagon to intraduodenal and intravenous administration of amino acids. J Clin Invest. 1968 Oct;47(10):2305–2322. doi: 10.1172/JCI105916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samols E., Tyler J., Marri G., Marks V. Stimulation of glucagon secretion by oral glucose. Lancet. 1965 Dec 18;2(7425):1257–1259. doi: 10.1016/s0140-6736(65)92278-6. [DOI] [PubMed] [Google Scholar]
- Siperstein M. D., Unger R. H., Madison L. L. Studies of muscle capillary basement membranes in normal subjects, diabetic, and prediabetic patients. J Clin Invest. 1968 Sep;47(9):1973–1999. doi: 10.1172/JCI105886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M., MADISON L. L. The effects of total starvation upon the levels of circulating glucagon and insulin in man. J Clin Invest. 1963 Jul;42:1031–1039. doi: 10.1172/JCI104788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M., McCALL M. S., KELLER S., LANZ H. C., MADISON L. L. Glucagon antibodies and their use for immunoassay for glucagon. Proc Soc Exp Biol Med. 1959 Oct-Dec;102:621–623. doi: 10.3181/00379727-102-25338. [DOI] [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M., McCALL M. S., MADISON L. L. Glucagon antibodies and an immunoassay for glucagon. J Clin Invest. 1961 Jul;40:1280–1289. doi: 10.1172/JCI104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M., McCALL M. S., MADISON L. L. Measurements of endogenous glucagon in plasma and the influence of blood glucose concentration upon its secretion. J Clin Invest. 1962 Apr;41:682–689. doi: 10.1172/JCI104525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Ketterer H., Dupré J., Eisentraut A. M. The effects of secretin, pancreozymin, and gastrin on insulin and glucagon secretion in anesthetized dogs. J Clin Invest. 1967 Apr;46(4):630–645. doi: 10.1172/JCI105565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Ohneda A., Aguilar-Parada E., Eisentraut A. M. The role of aminogenic glucagon secretion in blood glucose homeostasis. J Clin Invest. 1969 May;48(5):810–822. doi: 10.1172/JCI106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Ohneda A., Valverde I., Eisentraut A. M., Exton J. Characterization of the responses of circulating glucagon-like immunoreactivity to intraduodenal and intravenous administration of glucose. J Clin Invest. 1968 Jan;47(1):48–65. doi: 10.1172/JCI105714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]


